Please help us feed hungry people by improving dairy cattle in developing countries.
Dr. Wheeler is recipient of a Campus Award for Excellence in Graduate Student Mentoring
CHAMPAIGN, Ill. — The University of Illinois Urbana-Champaign each year presents Campus Awards for Excellence in Instruction to exceptional faculty and staff members, graduate teaching assistants and advisors campuswide. This year’s recipients are being honored at a ceremony.
Awardees are cited for sustained excellence and innovation in undergraduate and graduate teaching, undergraduate and graduate advising and mentoring, online teaching and research guidance. The Office of the Provost sponsors the awards.

Matthew B. Wheeler
Photo by Michelle Hassel
Matthew B. Wheeler, a professor of animal sciences, and Qian Chen, a professor of materials science and engineering, are recipients of the Campus Award for Excellence in Graduate Student Mentoring, presented to tenure-system or specialized faculty members at Illinois who have taught on the Urbana-Champaign campus for at least five years. According to their nominators:
Wheeler adopts a hands-on approach to graduate teaching and mentoring. The graduate-level course he taught for many years on advanced animal reproductive physiology allowed students to experience outside the classroom what they learned in lectures. Wheeler is a quintessential professor for a land-grant university, training the next generation of scientists while seamlessly connecting his research program with teaching and outreach activities. He has sustained dedication and enthusiasm for graduate education in reproductive biology throughout his career.
Milk to the rescue for diabetics? Illinois project creates first insulin-producing cow
March 13, 2024 Lauren Quinn ANSC / Health / Livestock / Genetics

Matt Wheeler, pictured, helped develop the first transgenic cow to produce human insulin in her milk.
An unassuming brown bovine from the south of Brazil has made history as the first transgenic cow capable of producing human insulin in her milk. The advancement, led by researchers from the University of Illinois Urbana-Champaign and the Universidade de Sao Paulo, could herald a new era in insulin production, one day eliminating drug scarcity and high costs for people living with diabetes.
“Mother Nature designed the mammary gland as a factory to make protein really, really efficiently. We can take advantage of that system to produce a protein that can help hundreds of millions of people worldwide,” said Matt Wheeler, professor in the Department of Animal Sciences, part of the College of Agricultural, Consumer and Environmental Sciences (ACES) at U. of I. He is also affiliated with the Carle Illinois College of Medicine, The Grainger College of Engineering, the College of Veterinary Medicine, the Beckman Institute, and the Carl R. Woese Institute for Genomic Biology.
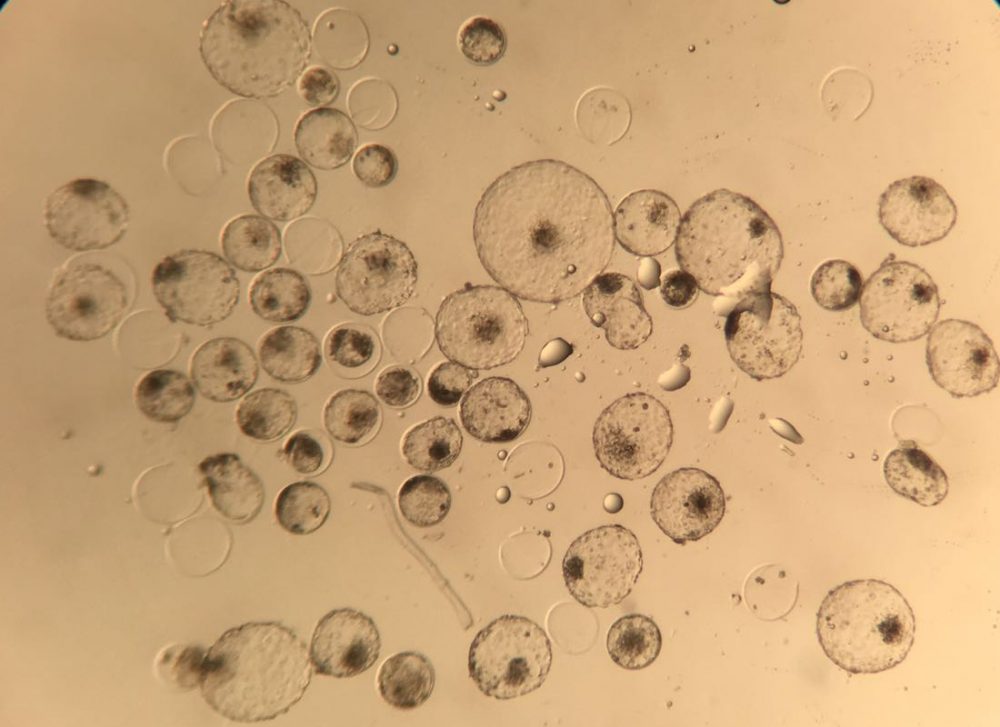
Wheeler is lead author on a new Biotechnology Journal study describing the development of the insulin-producing cow, a proof-of-concept achievement that could be scaled up aWer additional testing and FDA approval. Wheelerʼs colleagues in Brazil inserted a segment of human DNA coding for proinsulin — the protein precursor of the active form of insulin — into cell nuclei of 10 cow embryos. These were implanted in the uteruses of normal cows in Brazil, and one transgenic calf was born. Thanks to updated genetic engineering technology, the human DNA was targeted for expression — the process whereby gene sequences are read and translated into protein products — in mammary tissue only.
“In the old days, we used to just slam DNA in and hope it got expressed where you wanted it to,” Wheeler said. “We can be much more strategic and targeted these days. Using a DNA construct specific to mammary tissue means thereʼs no human insulin circulating in the cowʼs blood or other tissues. It also takes advantage of the mammary glandʼs capabilities for producing large quantities of protein.”
When the cow reached maturity, the team unsuccessfully attempted to impregnate her using standard artificial insemination techniques. Instead, they stimulated her first lactation using hormones. The lactation yielded milk, but a smaller quantity than would occur after a successful pregnancy. Still, human proinsulin and, surprisingly, insulin were detectable in the milk.
“Our goal was to make proinsulin, purify it out to insulin, and go from there. But the cow basically processed it herself. She makes about three to one biologically active insulin to proinsulin,” Wheeler said. “The mammary gland is a magical thing.”
The insulin and proinsulin, which would need to be extracted and purified for use, were expressed at a few grams per liter in the milk. But because the lactation was induced hormonally and the milk volume was smaller than expected, the team canʼt say exactly how much insulin would be made in a typical lactation.
Conservatively, Wheeler says if a cow could make 1 gram of insulin per liter and a typical Holstein makes 40 to 50 liters per day, thatʼs a lot of insulin. Especially since the typical unit of insulin equals 0.0347 milligrams. “That means each gram is equivalent to 28,818 units of insulin,” Wheeler said. “And that’s just one liter; Holsteins can produce 50 liters per day. You can do the math.”
The team plans to re-clone the cow, and is optimistic theyʼll achieve greater success with pregnancy and full lactation cycles in the next generation. Eventually, they hope to create transgenic bulls to mate with the females, creating transgenic offspring that can be used to establish a purpose-built herd. Wheeler says even a small herd could quickly outcompete existing methods — transgenic yeast and bacteria — for producing insulin, and could do so without having to create highly technical facilities or infrastructure.
“With regard to mass-producing insulin in milk, youʼd need specialized, high-health-status facilities for the cattle, but itʼs nothing too out of the ordinary for our well-established dairy industry,” Wheeler said. “We know what weʼre doing with cows.”
An efficient system to collect and purify insulin products would be needed, as well as FDA approval, before transgenic cows could supply insulin for the worldʼs diabetics. But Wheeler is confident that day is coming. “I could see a future where a 100-head herd, equivalent to a small Illinois or Wisconsin dairy, could produce all the insulin needed for the country,” he said. “And a larger herd? You could make the whole worldʼs supply in a year.
The study, “Human proinsulin production in the milk of transgenic cattle,” is published in Biotechnology Journal [DOI: 10.1002/biot.202300307]. The research was supported by the National Council for Scientific and Technological Development, CNPq [grant no. 245886/2012-5]; the University of Northern Parana, UNOPAR; and the USDA National Institute of Food and Agricultureʼs Multistate Research Fund [project no. W-4171].
Story Source(s): Matt Wheeler
ACES NEWS
Beth Bangert wins IETS CANDES Committee Travel Award.
Beth Bangert (left), M.S. student in Dr. Wheeler’s Group won an IETS CANDES Committee Travel Award to present her research entitled, “The efficiency of an adapted beef cattle protocol to produce in vitro-derived embryos from oocytes collected via surgical ovum pick-up from live white-tailed deer (Odocoileus virginianus) donors under captivity in central Illinois”, at the International Embryo Technology Society’s 50th Anniversary Annual Conference in Denver, Colorado on January 9, 2024.

Dr. Wheeler’s Group Presents Six Abstracts and Posters at the IETS’ 50th Anniversary Annual Conference in Denver, Colorado.

Dr. Wheeler’s Group Presents Six Abstracts and Posters at the IETS’ 50th Anniversary Annual Conference in Denver, Colorado. The abstracts were entitled:
Dr. Chanaka and Graduate Student Ken Wilson discuss a poster at the IETS Conference.
Bangert, E., Shipley, C., Rabel, R.A.C., Garrett, E., Milner, D. J., Marchioretto, P.V., Spencer, K., Allen, C., and Wheeler, M.B. (2023). The efficiency of an adapted bovine IVF protocol to produce in vitro-derived embryos from oocytes collected via surgical ovum pickup from live white-tailed deer (Odocoileus virginianus) donors under captivity in central Illinois. Reproduction, Fertility and Development 36, (2) 165-166 https://doi.org/10.1071/RDv36n2Ab32

Beth Bangert at her poster.
Glassey, J.R., Rabel, R.A.C., Milner, D.J. and Wheeler, M.B. (2023) Strontium enhances in vitro osteogenic differentiation of porcine adipocyte–derived stem cells. Reproduction, Fertility and Development 36, (2) 265-266 https://doi.org/10.1071/RDv36n2Ab220
Dr. Chanaka Rabel discussing his and Joe Glassey’s poster with one of the IETS conference delegates.
Marchioretto, P.V., Rodriguez-Zas, S L., Womack, S.A., Lindsey, B.R., Milner, D.J., Rubessa, M., Wilson, K.C. and Wheeler, M.B. (2023). Effect of dominant follicle removal before ovum pickup in Girolando cattle. Reproduction, Fertility and Development 36, (2) 251-252 https://doi.org/10.1071/RDv36n2Ab193
Monzani, P.S., Sangalli, J.R., Sampaio, R.V., Guemra, S., Zanin, R., Adona, P.R., Berlingieri, M.A., Cunha Filho, L F.C., Mora-Ocampo, I.Y., Pirovani, C P., Meirelles, F.V., Ohashi, O. and Wheeler M.B. (2023). Human proinsulin and insulin production in the milk of transgenic cattle. Reproduction, Fertility and Development 36, (2) 231 https://doi.org/10.1071/RDv36n2Ab155
Womack, S.A., Bethke, E.B., King, W P., Milner, D.J., Rubessa, M., Marchioretto, P.V., and Wheeler, M.B. (2023). Development of a porcine model for the testing of the RapidVent emergency ventilator for the treatment of COVID-19 infection. Reproduction, Fertility and Development 36, (2) 219 https://doi.org/10.1071/RDv36n2Ab132
Zimmerman, L.A., Bangert, E. A., Rabel, R. A C., Milner, D. J., Marchioretto, P.V., Allen, C.A. and Wheeler, M.B. (2023). The in vitroproduction of Gyr X Jersey bovine embryos from oocytes collected via ovum pickup for use in the tropics. Reproduction, Fertility and Development 36, (2) 155 https://doi.org/10.1071/RDv36n2Ab12

Luke Zimmerman standing by his poster at IETS
Dr. Wheeler leads Pre-Conference Symposium at the International Embryo Technology Society’s 50th Anniversary Annual Conference in Denver, Colorado.
Dr. Wheeler leads Pre-Conference Symposium at the International Embryo Technology Society’s 50th Anniversary Annual Conference in Denver, Colorado.
The IETS Pre-Conference Symposium 2024
Communicating and Demystifying Bovine Embryo Assisted Reproductive Technologies
Sponsored by WTA Technologies LLC, University of Illinois, Colorado State University, AETA, and
Ovitra Biotechnologies.


UI researchers to launch genetically improved cow project in Tanzania
The Daily Illini • December 30, 2023 • https://dailyillini.com/news-stories/around-campus/2023/12/30/ui-gmo-cow-project-tanzania/

Isaac Pinkus
A cow stands at her water basin at the Dairy Cattle Research Unit on South Lincoln Dr. and Hazelwood Dr. on Nov. 11.
By Megan Tafalla, Contributing Writer
In March 2024, animal scientists from the University will travel to Tanzania to launch a pilot project of cattle that will produce 10 times more milk than indigenous Tanzanian cattle.
The Tanzania Tropical Adapted Cattle Project, led by Matthew Wheeler, professor in ACES, crossbreeds Gyr cattle and Jersey cattle to create a hybrid dairy cow.
The hybrid’s hollow hairs and increased skin area, taken from the Gyr, will allow it to endure Tanzania’s tropical heat. Jersey genetics will give the hybrid longer lactation periods and larger udders, allowing it to produce an average of 5 liters of milk per day.
In comparison, indigenous Tanzanian cattle produce half a liter per day.
The team will begin the first phase of the project at two pilot locations in Tanzania. Before the trip, they’ll inseminate 100 Jersey embryos with Gyr semen, then freeze the embryos for transportation. Then, in Tanzania, they’ll insert the embryos into indigenous Tanzanian cattle.
Once the indigenous cattle give birth to the Jersey-Gyr hybrids, the team will remove embryos from the hybrids and repeat the insemination process until the fifth generation of Jersey-Gyr hybrids.
“The reason you have to do that is because of the genetics,” Wheeler said. “Every time you breed them, the DNA recombines. And by the time you get to the pure synthetic generation, the genes are fixed. So at that generation, we can actually select for higher milk production.”
Accounting for the crossbreeding process, it will take 10 years to reach the fifth generation of cattle.
The team will collaborate with the Maasai, a pastoral and semi-nomadic tribe in Kenya and Tanzania who depend on cattle.
In addition to optimizing milk production, the team will introduce new cattle management strategies and embryonic crossbreeding technologies to the Maasai to optimize the Jersey-Gyr hybrid’s milk production. Furthermore, the creation of a cattle genetic record system will help the herdsmen to track their animals’ genetics.
Researchers from China, the Philippines, Jamaica and Uganda have also contacted the team for work.
“I decided that what I’d like to do with the rest of my career is try to get these animals, a high health status herd of these animals, and get the genetics disseminated to … the Global South and try to help feed people,” Wheeler said. “Because really, when you sign up to be in agriculture, you sign up to feed people.”
CORRECTION Jan. 25, 11:22 p.m.: A previous version of this article was inaccurately titled “UI researchers to launch GMO cow project in Tanzania.” The official definition of a GMO, per the U.S. Department of Agriculture, is “a plant, animal or microbe in which one or more changes have been made to the genome, typically using high-tech genetic engineering, in an attempt to alter the characteristics of an organism.” As the cattle in this project do not fall under this definition, the title of this article has since been corrected.
Climate-smart cows could deliver 10-20x more milk in Global South
ACES NEWS
Explore News by:
October 31, 2023
Lauren Quinn
ANSC Livestock Breeding International

Herd of quarter-Holstein, three-quarter-Gyr cattle
URBANA, Ill. — A team of animal scientists from the University of Illinois Urbana-Champaign is set to deliver a potential game changer for subsistence farmers in Tanzania: cows that produce up to 20 times the milk of indigenous breeds.
The effort, published in Animal Frontiers, marries the milk-producing prowess of Holsteins and Jerseys with the heat, drought, and disease-resistance of Gyrs, an indigenous cattle breed common in tropical countries. Five generations of crosses result in cattle capable of producing 10 liters of milk per day under typical Tanzanian management, blasting past the half-liter average yield of indigenous cattle.
After breeding the first of these calves in the U.S., project leader Matt Wheeler, professor in the Department of Animal Sciences in the College of Agricultural, Consumer and Environmental Sciences (ACES) at Illinois, is ready to bring embryos to Tanzania.
“High-yielding Girolandos — Holstein-Gyr crosses — are common in Brazil, but because of endemic diseases there, those cattle can’t be exported to most other countries,” Wheeler said. “We wanted to develop a high health-status herd in the U.S. so we could export their genetics anywhere in the world.”
Wheeler’s team plans to implant 100 half-blood Holstein-Gyr or Jersey-Gyr embryos into indigenous cattle in two Tanzanian locations this March. The resulting calves will be inseminated through successive generations to create “pure synthetic” cattle with five-eighths Holstein or Jersey and three-eighths Gyr genetics. Unlike Girolandos, Jersey-Gyr pure synthetics do not yet have an official name.

Half-blood Holstein x Gyr calf
Pure synthetics are worth the time and effort; once the five-eighths/three-eighths genetics are established, they’re locked in. In other words, calves from successive matings will maintain the same genetic ratio.
“The whole idea is to keep the disease and pest resistance linked together with the milk production so that as you breed, those traits don’t separate,” Wheeler said. “That’s going to be the challenge in developing countries; until you get to the pure synthetic generation, there will always be the temptation to breed to the bull down the road, losing the effect.”
Wheeler’s team, including coauthor Moses Ole-Neselle of the Food and Agriculture Organization of the United Nations (FAO), cares about getting this effort right. Although developing the embryos took years of meticulous work, they’re not stopping there. The team hosted its first online course on bovine assisted reproduction technology last summer, including 12 participants from Tanzania. And there’s more to come.
“It was important to start training the first group of veterinarians and graduate students to adopt the technology, so when we get there, it’s not a foreign thing,” Wheeler said. “The Tanzanian government wants this training and student exchanges. We’re going to continue investing in this program for as long as it takes.”
Wheeler recognizes the best genetics and most comprehensive training won’t amount to much if the plan doesn’t account for the local culture. With advice from collaborators like the Tanzania Livestock Research Institute and Teresa Barnes, director of the Center for African Studies at Illinois, Wheeler has already adjusted his strategy to accommodate the preferences of local Maasai herdsmen.
“We’ve learned some Maasai clans strongly prefer smaller, red cattle, so the Holstein crosses we made initially, which were large and black, weren’t going to work,” he said. “I had to start over with Jerseys, which set us back a bit. It will be worth it if they’re better accepted.”
But some aspects of Tanzanian cattle management will have to change to realize the full potential of the improved genetics. For example, Wheeler said nomadic Maasai herders often graze cattle 25 miles from their enclosures each day, limiting the energy available for milk production.
While the project is still in its early stages, it represents a step toward more climate-resilient animal agriculture, the topic of the special issue of Animal Frontiers in which Wheeler’s article is published. While Wheeler’s current priority is to bolster food security in the Global South where climate change is hitting hardest, he said the same technology could be used to protect cattle from changing climates here in the U.S. and around the world. In other words, tropical genetics could be inserted into our already high-yielding cattle to better withstand heat, drought, and disease.
“These cattle would work very well in Mexico, Texas, New Mexico, and California. Maybe it’s time to start thinking about that now,” Wheeler said. “People don’t usually think that far ahead, but my prediction is that people are going to look back and realize having tropical genetics earlier would have been a good thing.”
The study, “Development of genetically improved tropical-adapted dairy cattle,” is published in Animal Frontiers [DOI:10.1093/af/vfad050]. Authors include Paula Marchioretto, Chanaka Rabel, Crystal Allen, Moses Ole-Neselle, and Matthew B. Wheeler. This work was partially supported by the USDA Multistate Project W-4171, the ACES Office of International Programs, the University of Illinois National Resource Center in African Studies via funding through the Department of Education’s Title VI grant program for 2022-26 and the Ross Foundation (Agreement #635148).
Story Source(s)
Dr. Wheeler presented with the 2023 Spitze Land-Grant Professorial Career Excellence Award sponsored by the College of Agricultural, Consumer and Environmental Sciences.
Image

This award is presented to recognize the professorial career of tenured faculty in their performance and commitment to teaching and advising; research and publications; extension and public service; faculty governance; and participation in professional associations. The award, which was first presented in 2003, also memorializes the unique mission and rich history of the public land-grant university and exemplifies the continued opportunities it offers for worldwide contribution to the betterment of humankind and to democratic institutions in an ever-changing environment.
Tropical-Adapted Dairy Cattle Project Published in Animal Frontiers

Development of Genetically Improved Tropical-Adapted Dairy Cattle” Animal Frontiers, Volume 13, Issue 5, October 2023, Pages 7–15, https://doi.org/10.1093/af/vfad050