Molecular Force Measurements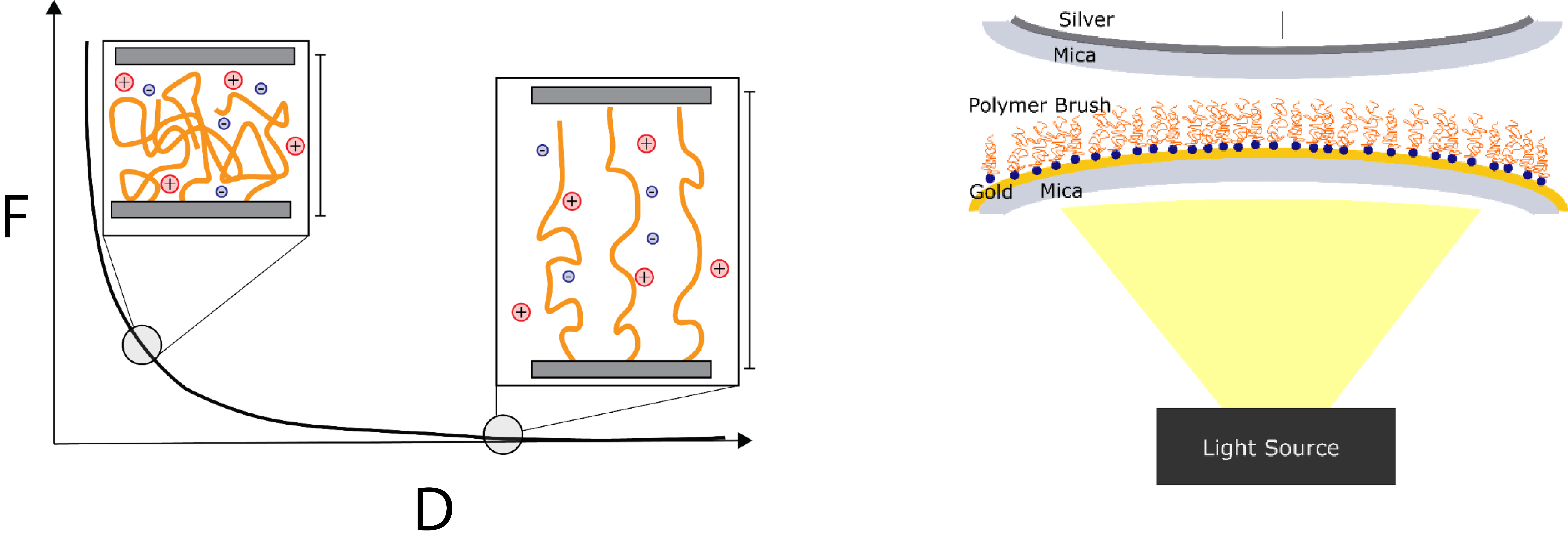
Nano scale force measurements are used to quantify interfacial force fields that control biomaterial performance in medical applications. Studies focus on protein-compatible, water-soluble polymers used in drug delivery, tissue engineering, and biosensor coatings. Surface force apparatus (SFA) and colloidal probe atomic force microscopy (CP-AFM) measurements directly quantify force fields within 0.1-100 nm of material surfaces. Current studies of Zwitterionic polymers (e.g. poly sulfobetaine) are identifying molecular design rules underlying the unique properties of these materials.
Biomaterial Effects on Protein Folding
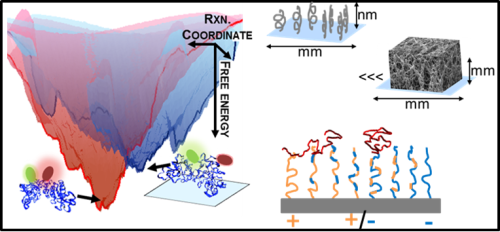
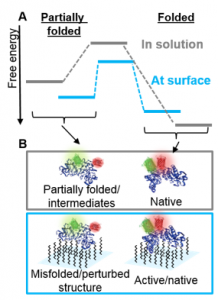
The diversity of protein functions has inspired their use in hybrid bio-materials including biosensors, pharmaceuticals, tissue engineering, and drug targeting. Yet incorporating proteins in these abiotic environments can degrade their stability and function. Currently, few approaches can determine how material environments alter protein stability.
We are developing Fast Relaxation Imaging (FReI) in collaboration with the Gruebele research group to define material design rules that preserve protein stability and function. FReI was developed to study protein folding in cells, but has untapped potential to interroga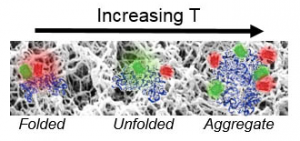 te proteins in materials at sub micron resolution. We are extending the FReI approach to image protein folding stability material. Studies of proteins in a “library” of materials are identifying novel, nano scale design rules that preserve or shut down protein function.
te proteins in materials at sub micron resolution. We are extending the FReI approach to image protein folding stability material. Studies of proteins in a “library” of materials are identifying novel, nano scale design rules that preserve or shut down protein function.
We used FReI to determine how hydrogel–protein interactions and confinement in hydrogel nanopores alter protein folding stability.
Zwitterionic polymer-protein interactions
Poly(zwitterions) are polymers that contain equal numbers of cationic and anionic groups on each monomer. They have remarkable antii-fouling properties and have potential biomedical applications in wound dressings, biosensors, or stealth nanoparticles for drug delivery. Poly(zwitterion) performance in these applications is ascribed to polymer solvation, which is postulated to prevent protein adsorption.
 This raises the important questions: “Do poly(zwitterions) bind proteins?” and “How do the polymers affect protein stability?” We are studying poly(zwitterion) interactions with proteins both in solution and at surfaces. Fluorescence and biophysical measurements determine whether poly(zwitterions) bind proteins and the impact of protein-polymer interactions on protein stability and function. Our studies are identifying the physical chemical bases for the success or failure of poly(zwitterionic) materials in diverse applications.
This raises the important questions: “Do poly(zwitterions) bind proteins?” and “How do the polymers affect protein stability?” We are studying poly(zwitterion) interactions with proteins both in solution and at surfaces. Fluorescence and biophysical measurements determine whether poly(zwitterions) bind proteins and the impact of protein-polymer interactions on protein stability and function. Our studies are identifying the physical chemical bases for the success or failure of poly(zwitterionic) materials in diverse applications.