 Ravishankar K. Iyer
Ravishankar K. Iyer
Department of Electrical and Computer Engineering
University of Illinois at Urbana-Champaign
rkiyer@illinois.edu
Electronic and computer-based medical devices are being widely deployed in various clinical and personalized settings, but increased device complexity raises major challenges in ensuring device reliability and patient safety. During 2007−2013, about 16,000 recalls and over 2.4 million device-associated adverse events were reported to the U.S. Food and Drug Administration (FDA). Study of medical device incidents provides valuable insights into device safety issues and also into how their designs could be improved to prevent catastrophic patient impacts in the future. 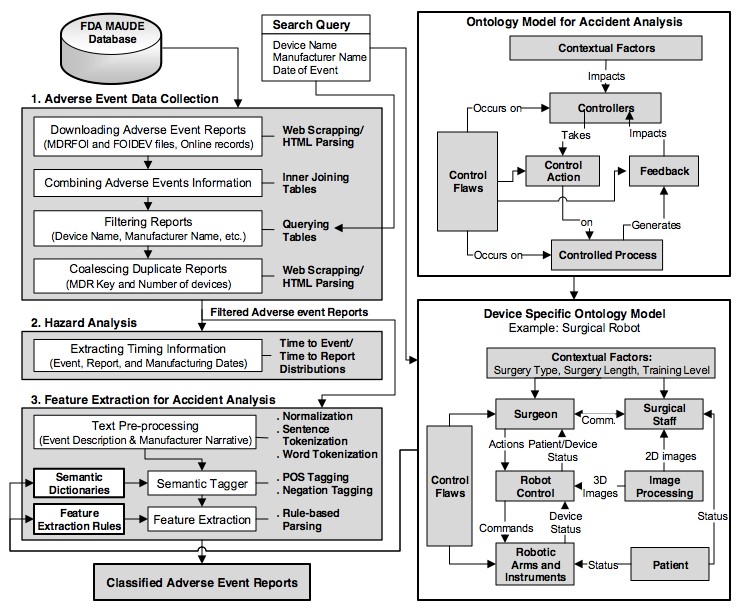 Existing techniques used in accident analysis and reporting are often inadequate for understanding the causes of incidents in today’s complex medical systems. We present our research on discovering the causes of medical device failures and their impact on patients, by analyzing the FDA data on recalls and adverse event reports. We propose a new control-theoretic causality model for analysis of the multidimensional factors leading to complex medical device accidents, exemplified by surgical robots. The proposed model can also be used to drive the design of real-time safety monitors and accident data collection and mining techniques for the next generation of medical systems that are inherently safe while mitigating catastrophic device failures as well as accidental operator errors.
Existing techniques used in accident analysis and reporting are often inadequate for understanding the causes of incidents in today’s complex medical systems. We present our research on discovering the causes of medical device failures and their impact on patients, by analyzing the FDA data on recalls and adverse event reports. We propose a new control-theoretic causality model for analysis of the multidimensional factors leading to complex medical device accidents, exemplified by surgical robots. The proposed model can also be used to drive the design of real-time safety monitors and accident data collection and mining techniques for the next generation of medical systems that are inherently safe while mitigating catastrophic device failures as well as accidental operator errors.
References:
- Alemzadeh, J. Raman, N. Leveson, R. K. Iyer, “Safety Implications of Robotic Surgery: A Study of 13 Years of FDA Data on da Vinci Surgical Systems,” J. Maxwell Chamberlain Memorial Paper in Adult Cardiac Surgery, Proc. of the 50th Annual Meeting of the Society of Thoracic Surgeons (STS), Jan. 2014. CSL Technical Report, UILU-ENG-13-2208, Nov. 2013.
- Alemzadeh, R. Hoagland, Z. Kalbarczyk, R. K. Iyer, “Automated Classification of Computer-based Medical Device Recalls,” Proc. of the 27th IEEE International Symposium on Computer-Based Medical Systems (CBMS 2014), May 2014.
- Alemzadeh, R. K. Iyer, Z. Kalbarczyk, J. Raman, “Analysis of Safety-Critical Computer Failures in Medical Devices,” IEEE Security & Privacy, vol. 11, no. 4, pp. 14-26, July-Aug. 2013.
Ravi K. Iyer is the George and Ann Fisher Distinguished Professor of Engineering at the University of Illinois at Urbana-Champaign. He holds joint appointments in the Department of Electrical and Computer Engineering, the Coordinated Science Laboratory (CSL), and the Department of Computer Science and serves as Chief Scientist of the Information Trust Institute. Iyer has led several large successful projects funded by NASA, DARPA, NSF, and industry. He currently co-leads the CompGen Center at Illinois. Funded by NSF and partnering with industry leaders, hospitals, and research labs, CompGen aims to build a new computational platform to address both accuracy and performance issues for a range of genomics applications. Professor Iyer is a Fellow of the American Association for the Advancement of Science, the IEEE, and the ACM. He has received several awards, including the AIAA (American Institute for Aeronautics and Astronautics) Information Systems Award, the IEEE Emanuel R. Piore Award, and the 2011 Outstanding Contributions award from the Association of Computing Machinery’s Special Interest Group on Security for his fundamental and far-reaching contributions in secure and dependable computing. Professor Iyer is also the recipient of the degree of Doctor Honaris Causa from Toulouse Sabatier University in France.