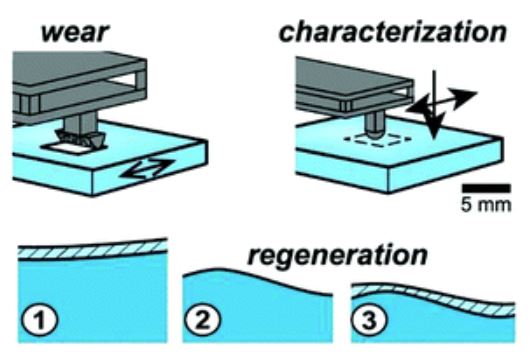
We have sought the answer to this question for years! In fact, I started working with contact lenses as an undergraduate at Florida in Greg Sawyer’s lab. Our answer at the time was “chemistry.” Our answer through the years shifted to consider structured surface layers, polymer fluctuation, and hydration, among others. But it is just now that we are finally able to explain. The answer lies not only in chemistry, but in the water/polymer interactions internal to the hydrogel: the osmotic pressure. Consider: inside a hydrophilic gel is an osmotic pressure which seeks to pull water in, and swell it; outside the gel this pressure is zero. Pressure cannot have a discontinuity, and as such the surface must swell slightly to create the osmotic pressure gradient, and a less dense, softer surface!
I’m thrilled to say that this paper has just come out in Soft Matter: “Self-regenerating compliance and lubrication of polyacrylamide hydrogels.” The hard work was done by Shabnam Bonyadi to get this thing out. Send us an email! Ask me anything!