 Microbial Conversion of Glucocorticoids into Androgens
Microbial Conversion of Glucocorticoids into Androgens
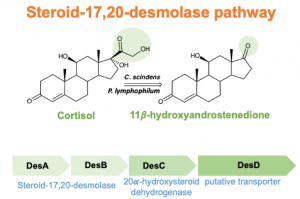
Human and animal microbiomes are capable of extensive modification of host steroids. One reaction of particular interest to our lab is known as steroid-17,20-desmolase, an enzyme encoded by the desAB genes. The DesAB enzyme catalyzes the side-chain cleavage of cortisol and its derivatives resulting in the formation of precursors to 11-oxy-androgens. The conversion of glucocorticoids to androgens in the GI tract may be significant because epithelial and immune cells in the gut express nuclear androgen receptor. Our lab also determined that isolates from the urinary tract encode desAB and convert cortisol and its derivatives to 11-oxy-androgens. An accumulating body of evidence indicates that 11-oxy-androgens are important in prostate cancer development and progression. We are working towards understanding the effects of bacterial steroid-17,20-desmolase in the GI tract in several experimental models. Clinical studies on the role of bacterial steroid-17,20-desmolase in colorectal cancer and prostate cancer are currently underway.
Side-chain Oxidation
We previously showed that the desC gene from C. scindens ATCC 35704 encodes an NADH-dependent 20α-hydroxysteroid dehydrogenase, and recently we have performed extensive structural, biochemical, and theoretical studies on the DesC, a Zn-dependent medium chain dehydrogenase. The desC gene is co-expressed with desAB genes, and functions to convert cortisol to 20α-dihydrocortisol. The host also produces enzyme(s) that generate 20α-dihydrocortisol, a metabolite enriched in Cushing’s Syndrome, in which excess cortisol secretion is observed. Prior literature also identified Butyricicoccus desmolans and Clostridium cadavaris as capable of cortisol side-chain cleavage or cortisol, as well as the ability to produce the stereoisomer of 20α-dihydrocortisol, known as 20β-dihydrocortisol. We located the desAB genes in B. desmolans and C. cadavaris, as well as a gene encoding a predicted short chain dehydrogenase that we hypothesized was a 20β-hydroxysteroid dehydrogenase. Indeed, we determined that this enzyme was capable of converting cortisol to 20β-dihydrocortisol in the presence of NADH. We named the gene, desE, and determined that desE was in diverse gut bacteria including bifidobacteria. We crystalized the DesE from Bifidobacterium adolescentis and biochemically characterized the active site amino acid residues. We are working towards understanding the effects of bacterial 20α-dihydrocortisol and 20β-hydroxysteroid dehydrogenase in the GI tract in several experimental models.
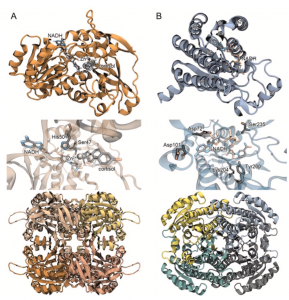
A. Structure of DesC from Clostridium scindens ATCC 35704. Top: Monomer with ligands (NADH, cortisol). Middle: Active-Site with NADH bound. Bottom: Quaternary Structure (homotetramer). B. Structure of DesE from Bifidobacterium adolescentis. Top: Monomer with ligand (NADH). Middle: Active-Site with NADH bound. Bottom: Quaternary Structure (homotetramer).
Steroid Metabolism Publications
- Doden HL, Ridlon JM. Microbial Hydroxysteroid Dehydrogenases: From Alpha to Omega. Microorganisms. 2021 Feb 24;9(3):469. doi: 10.3390/microorganisms9030469. PMID: 33668351; PMCID: PMC7996314.
- Ly LK, Doden HL, Ridlon JM. Gut feelings about bacterial steroid-17,20-desmolase. Mol Cell Endocrinol. 2021 Apr 5;525:111174. doi: 10.1016/j.mce.2021.111174. Epub 2021 Jan 24. PMID: 33503463.
- Ly LK, Rowles JL 3rd, Paul HM, Alves JMP, Yemm C, Wolf PM, Devendran S, Hudson ME, Morris DJ, Erdman JW Jr, Ridlon JM. Bacterial steroid-17,20-desmolase is a taxonomically rare enzymatic pathway that converts prednisone to 1,4-androstanediene-3,11,17-trione, a metabolite that causes proliferation of prostate cancer cells. J Steroid Biochem Mol Biol. 2020 May;199:105567. doi: 10.1016/j.jsbmb.2019.105567. Epub 2019 Dec 20. PMID: 31870912; PMCID: PMC7333170.
- Ridlon JM. Conceptualizing the Vertebrate Sterolbiome. Appl Environ Microbiol. 2020 Aug 3;86(16):e00641-20. doi: 10.1128/AEM.00641-20. PMID: 32503912; PMCID: PMC7414951.
-
Xavier JB, Young VB, Skufca J, Ginty F, Testerman T, Pearson AT, Macklin P, Mitchell A, Shmulevich I, Xie L, Caporaso JG, Crandall KA, Simone NL, Godoy-Vitorino F, Griffin TJ, Whiteson KL, Gustafson HH, Slade DJ, Schmidt TM, Walther-Antonio MRS, Korem T, Webb-Robertson BM, Styczynski MP, Johnson WE, Jobin C, Ridlon JM, Koh AY, Yu M, Kelly L, Wargo JA. The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer. 2020 Mar;6(3):192-204. doi: 10.1016/j.trecan.2020.01.004. Epub 2020 Feb 7. PMID: 32101723; PMCID: PMC7098063.
-
Doden HL, Pollet RM, Mythen SM, Wawrzak Z, Devendran S, Cann I, Koropatkin NM, Ridlon JM. Structural and biochemical characterization of 20β-hydroxysteroid dehydrogenase from Bifidobacterium adolescentis strain L2-32. J Biol Chem. 2019 Aug 9;294(32):12040-12053. doi: 10.1074/jbc.RA119.009390. Epub 2019 Jun 17. PMID: 31209107; PMCID: PMC6690682.
-
Devendran S, Mythen SM, Ridlon JM. The desA and desB genes from Clostridium scindens ATCC 35704 encode steroid-17,20-desmolase. J Lipid Res. 2018 Jun;59(6):1005-1014. doi: 10.1194/jlr.M083949. Epub 2018 Mar 23. PMID: 29572237; PMCID: PMC5983398.
-
Morris DJ, Ridlon JM. Glucocorticoids and gut bacteria: “The GALF Hypothesis” in the metagenomic era. Steroids. 2017 Sep;125:1-13. doi: 10.1016/j.steroids.2017.06.002. Epub 2017 Jun 15. PMID: 28624548.
-
Devendran S, Méndez-García C, Ridlon JM. Identification and characterization of a 20β-HSDH from the anaerobic gut bacterium Butyricicoccus desmolans ATCC 43058. J Lipid Res. 2017 May;58(5):916-925. doi: 10.1194/jlr.M074914. Epub 2017 Mar 17. PMID: 28314858; PMCID: PMC5408610.
-
Ridlon JM, Ikegawa S, Alves JM, Zhou B, Kobayashi A, Iida T, Mitamura K, Tanabe G, Serrano M, De Guzman A, Cooper P, Buck GA, Hylemon PB. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J Lipid Res. 2013 Sep;54(9):2437-49. doi: 10.1194/jlr.M038869. Epub 2013 Jun 15. PMID: 23772041; PMCID: PMC3735941.

