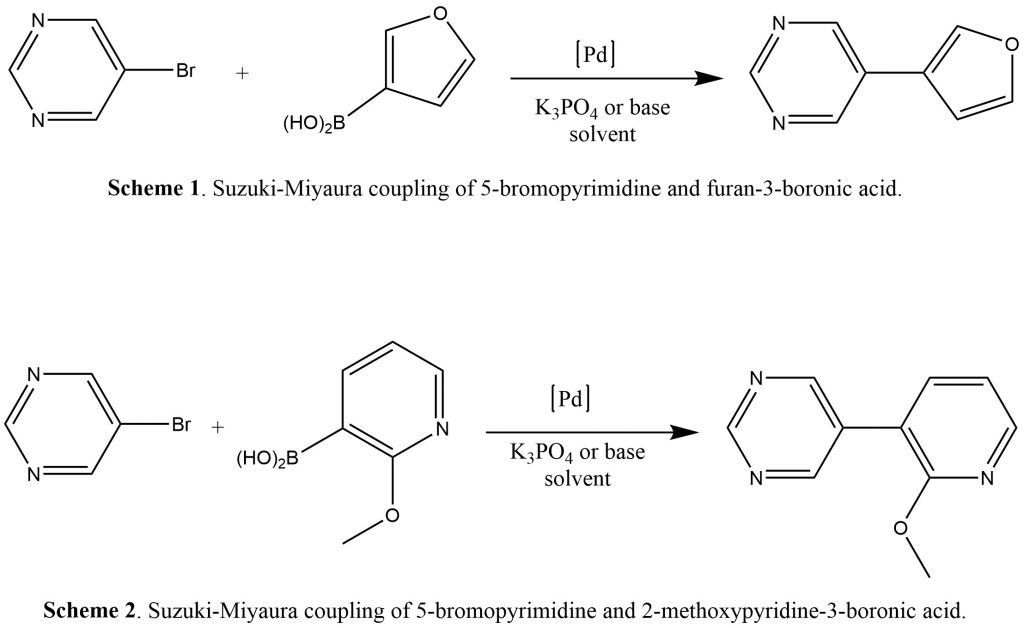
Abstract: “Suzuki-Miyaura (SM) coupling is a metal-catalyzed carbon-carbon bond reaction between organoboron and organohalide under basic conditions. Cross-coupling reactions are typically catalyzed by the expensive palladium (Pd) catalyst. This reaction is commonly used in the synthesis of pharmaceuticals, polymers, and agrochemicals. Our objectives include using the WebMO software in understanding the chemical and physical properties, and the energetics of the reaction between 5-bromopyrimidine and furan-3-boronic acid (Scheme 1) and the reaction between 5-bromopyrimidine and 2-methoxypyridine-3-boronic acid (Scheme 2), along with their products. Our calculations indicated that Schemes 1 and 2 are endothermic reactions, conforming to the well-known fact that connecting two sp2-hybridized carbons is a challenging task. The BDE data reveal that the strength of the C-X bond in the series of halobenzenes decreases with increasing size of the halogen. The C-X bond distances mirror this trend, with the longest bond (C-Br) also being the weakest. In the context of the oxidative addition step of a typical catalytic cycle for a Pd-catalyzed coupling reaction, It can be concluded that the C-Br bond is the most susceptible to oxidative addition. In the broader sense, the BDE data illustrate why aryl bromides are common starting materials for Pd-catalyzed couplings rather than the less expensive and environmentally more favorable aryl chlorides.”


Hey Alexis great talk, I had two general questions.
1) What is the mechanism of that oxidative addition step?
2) What kind of reactivity would you expect from an aryl iodide?
1) The mechanism will be as it is called/named. Pd(0) will be oxidized to Pd(II) and the aryl and halide will add to palladium.
2) The aryl iodides will be expected to be more reactive – these halides are actually the new systems of interest for recent development on cross-coupling reactions. A couple of literature are attached:
https://pubs.acs.org/doi/abs/10.1021/acs.orglett.7b00831
https://pubs.acs.org/doi/10.1021/acs.joc.6b01648
Very interesting presentation. You mentioned that you used WebMO to do the calculations, but what was the computational engine that was used, and the choices of basis set and functional if this was done using DFT methods? You also provided IR, NMR, and UV spectra for a compound, what was the significance of these spectra and how were they used in the determination of the best halide leaving group?
We used different calculation engines, theory, and basis sets to see the trends for the binding dissociation energy of different halide starting materials, heat of reactions for the 2 Suzuki-Miyaura cross coupling reactions, and steric hindrance of different substituents in the products. We looked for patterns and trends, realizing that each basis set and functionals have their own limitations. I just mentioned a set of results in my poster presentation which was the BDE calculation using Hartree-Fock theory and 3-21G basis set.
The IR, NMR, and UV-Vis were mainly additonal things that we did for characterization of the products. We looked at the ability of WebMO to do such calculations, as well as get the training needed to run those for reactions that we cannot perform in the lab.
Very clear slides, thank you. You said your aim was to investigate the first step of the mechanism, yet you used WebMO to calculate energy differences between the starting material and final product. If you wanted to investigate the first step, shouldn’t you be looking at the aryl halide and an aryl palladium species?
We used different calculation engines, theory, and basis sets to see the trends for the binding dissociation energy of different halide starting materials, heat of reactions for the 2 Suzuki-Miyaura cross coupling reactions, and steric hindrance of different substituents in the products. We looked for patterns and trends, realizing that each basis set and functionals have their own limitations. I just mentioned a set of results in my poster presentation which was the BDE calculation using Hartree-Fock theory and 3-21G basis set.
The IR, NMR, and UV-Vis were mainly additonal things that we did for characterization of the products. We looked at the ability of WebMO to do such calculations, as well as get the training needed to run those for reactions that we cannot perform in the lab.
I apologize for the previous response. It was supposed to post to the other comment.
To answer your question, yes, I looked at the aryl halides and reported those. All other calculations were done to further utilize WebMO, understand the whole cross coupling reactions that we did in the lab, and get training for future calculations needed for systems that we cannot run in the lab.
The comment regarding calculation of aryl palladium species is well-appreciated. We tried performing these calculations, which all failed. It is because of our WebMO license that cannot handle ones with metals. Upon WebMO upgrade, this is one of the main things that we wanted to pursue. Thank you.