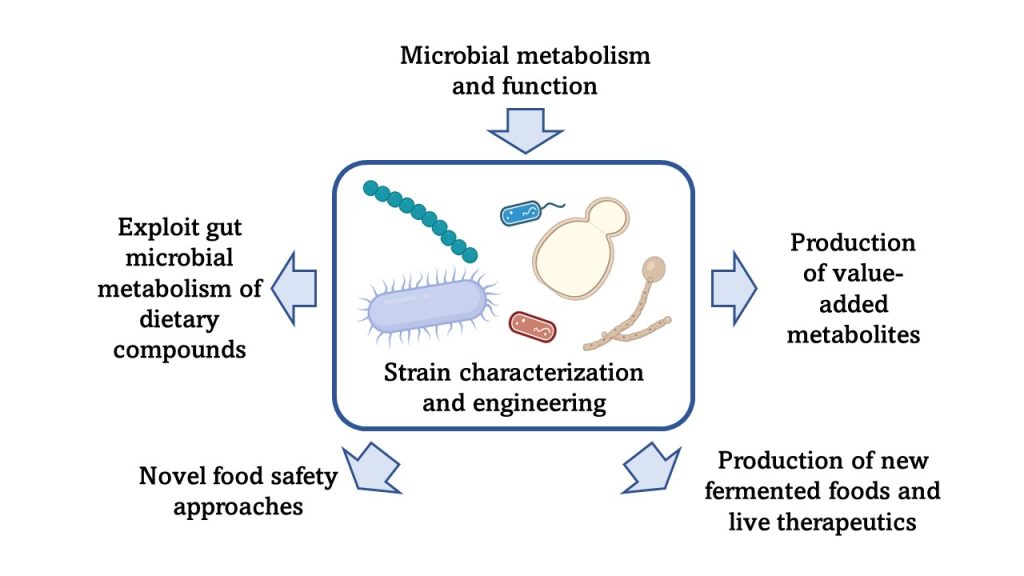
Current Projects
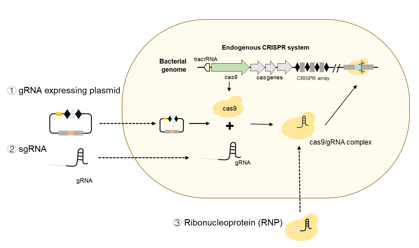
Development of genetic tools for stubborn Gram-positive bacteria
Some Gram-positive bacteria are notoriously difficult to genetically manipulate. Genetic manipulation is important for both associating phenotype with genotype and for genetic engineering. For example, developing a strain of LAB to produce more of a metabolite that is beneficial to human health would require the ability to manipulate the bacterial genome. There are a variety of well-established genetic tools for model systems (such as E. coli and S. cerevisiae) and the genetic toolbox is expanding to include many other lab strains. However, these tools do not work as well in some Gram-positive bacteria such as the LAB found in the gut or in fermented foods. Our goal is to fill this gap. We are doing this by two strategies: identifying new transformable plasmids, and developing a DNA-free transformable complex. Using CRISPR-Cas9 editing as the starting point, we are exploring new ways to edit stubborn genomes.
Impact of dietary brassica on host health
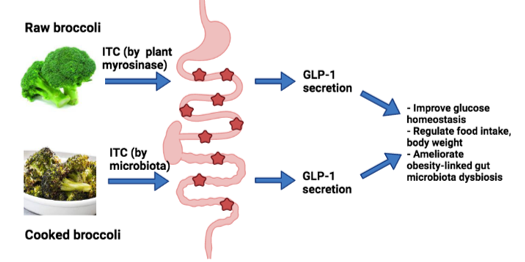
Summary of the grant proposal: We will examine interactions between brassica, gut microbiome (GM) and GLP-1 secretion. Brassica vegetables are a rich source of glucosinolates (GSLs), a group of sulfur compounds that are unique to these vegetables (broccoli, kale, and others). Upon hydrolysis, GSLs are converted to bioactive isothiocyanates (ITCs). When consumed raw, GSLs can be converted to ITCs by the plant enzyme myrosinase. Heat (cooking) inactivates myrosinase, then gut microbiota (GM) become responsible for any GSL conversion to ITCs. We hypothesize that non-ITC producing pathways present in the GM are competing for GSLs and thus explain the dramatic interindividual differences seen in ITC production (Aim 1). Once thought to be only present in the mouth, bitter-taste receptors (T2Rs) are now found to be present in many tissues including the gut. Since gut T2Rs can trigger GLP-1 secretion and ITCs can serve as T2R agonists, we hypothesize that ITCs produced by the GM bind intestinal T2R(s) to trigger GLP-1-dependent control of obesity and glucose homeostasis (Aim 2). Optimizing ITC production in the gut, both amount and location, has the potential to reverse glucose intolerance and obesity in humans. Our findings will inform the public about the health promoting properties of brassica, explain the variation in GSL bioactivation seen in human studies, where non-ITC metabolism of GSLs by GM have been little considered, and will further increase brassica intake worldwide.
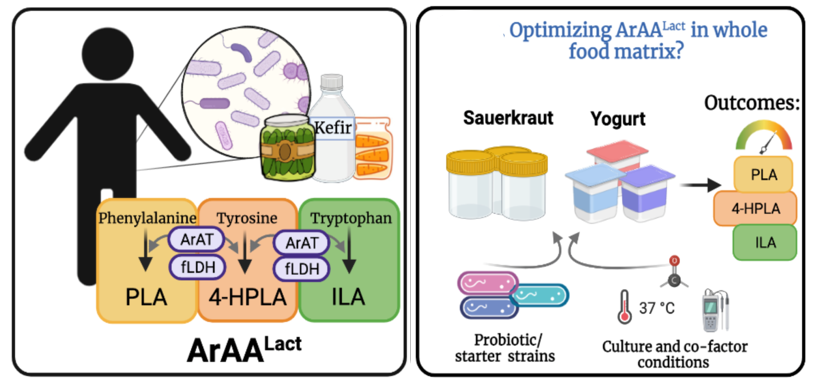
Optimizing bioactive metabolites in fermented foods to improve human immune function
Fermented food intake is associated with improvements in human immune function. These benefits may lie in bioactivity of microbe-derived metabolites (i.e. postbiotics) found in fermented foods. It is our vision that 1) identifying beneficial postbiotics and then 2) adapting strategies to optimize their production in a food matrix will have significant impact on immune health and disease prevention. Our preliminary data indicates that select fermented foods contain high concentrations of phenyllactic acid (PLA), 4-hydroxyphenyllactic acid (4-HPLA), and indole-3-lactic acid (ILA); biochemically related metabolites downstream of microbial aromatic amino acid (ArAA) metabolism that we have identified as mediators of innate immune cell (monocyte) inflammatory activity. These data have led us to hypothesize that there is a unique class of microbial products within fermented food that confers anti-inflammatory activity. However, how fermented food postbiotic properties can be optimized to improve immune health of humans has never been tested. To fill this gap in knowledge, we will: 1) Further investigate the immune and metabolic tuning properties of microbial-ArAA metabolites 2) Identify food matrix conditions that optimize microbial ArAA metabolism and 3) Determine if whole fermented food diets optimized for high microbial-derived ArAA metabolites promote anti-inflammatory activity in humans.
Development of improved fermented foods using previously engineered strains

This is our newest project in development! In collaboration with the Jin Lab, we are using previously engineered yeast and LAB strains from both labs to produce fermented food products and investigate the changes (and potential benefits) of the strains. This project involves the analysis of kombucha (a fermented tea beverage) in order to create a standard kombucha culture with engineered yeast so that future production can occur more quickly, more consistently, and without the reliance of a symbiotic colony (SCOBY). Future projects will involve research into the practicality of engineered yeast in the food industry for the production of clean-label dyes, preservatives, and flavorings.
Previous Work
To read about previous work done in the Miller Lab, please refer to the Publications page.