Advances in stem cell biology and materials science have provided a basis for developing tissue engineering methods to repair muscle injury. Among stem cell populations with potential to aid muscle repair, adipose-derived mesenchymal stem cells (ASC) hold great promise. To evaluate the possibility of using porcine ASC for muscle regeneration studies, we co-cultured porcine ASC with murine C2C12 myoblasts. These experiments demonstrated that porcine ASC display significant myogenic potential. Co-culture of ASC expressing green fluorescent protein (GFP) with C2C12 cells resulted in GFP+ myotube formation, indicating fusion of ASC with myoblasts to form myotubes. The presence of porcine lamin A/C positive nuclei in myotubes and RTqPCR analysis of porcine myogenin and desmin expression confirmed that myotube nuclei derived from ASC contribute to muscle gene expression. Co-culturing GFP+ASC with porcine satellite cells demonstrated enhanced myogenic capability of ASC, as the percentage of labeled myotubes increased compared to mouse co-cultures. Enhancing myogenic potential of ASC through soluble factor treatment or expansion of ASC with innate myogenic capacity should allow for their therapeutic use to regenerate muscle tissue lost to disease or injury.
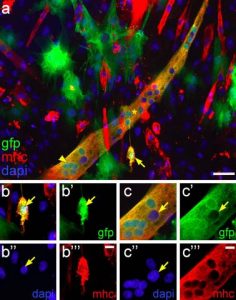
Pictured above: Culture in the presence of differentiating porcine satellite cells can initiate myogenesis in porcine ASC prior to fusion with myotubes. Co-cultures of porcine GFP+ ASC with porcine satellite cells undergoing myogenesis exhibit the presence of individual ASC expressing sarcomeric MHC. a Merged fluorescent images for GFP (green), sarcomeric MHC (red) and dapi (blue) clearly show an individual GFP+ mononuclear cell (arrow) expressing sarcomeric MHC, indicating that this is an ASC that has initiated the myogenic program. Also note the presence of a large GFP+ myotube (*) exhibiting clusters of GFP+ nuclei (arrowhead), indicating that this myotube has been formed with contribution of several ASC. b Enlarged single channel images and merged image of the mononuclear ASC marked by the arrow in (a). The arrow in each panel points to the single nucleus of the cell. Note the presence of GFP label in the nucleus. c Enlarged single channel images and merged image showing a cluster of GFP+ nuclei derived from ASC in the region marked by the arrowhead in (a). The arrow in each panel points to a nucleus derived from a porcine satellite cell lacking GFP
Derek J. Milner, Massimo Bionaz & Elisa Monaco, Jo Ann Cameron and Matthew B. Wheeler

