Our laboratory aims to understand how cells can be manipulated and engineered to facilitate targeted delivery of therapeutics and regulate intercellular interactions, in order to improve and innovate therapies for cancers, injured tissues, autoimmune disorders, and other diseases. In one path towards this goal, we utilize chemistry, chemical biology, and synthetic biology tools to modify or engineer cells for subsequent tracking and targeted modulation in vivo. In another path, we develop biomaterials that can home and manipulate immune cells in vivo, and apply them to the development of cancer vaccines, cell therapies, and medical devices. New molecules, polymers, and biomaterials that can serve as diagnostics, therapeutics, and medical supplies/devices are also of our interest.
1. Metabolic Labeling and Targeting of Cells
Cells can actively metabolize unnatural monosaccharides bearing chemical tags and express them in the form of glycoproteins. These cell-surface chemical tags (e.g., azide) enable targeted conjugation of molecules of interest via efficient chemistries to monitor or modulate cells. In the context of cancer cells, we have been exploring cancer-selective labeling in vivo and subsequent development of cancer-targeted therapies. Similarly, unnatural sugars can metabolically label immune cells with chemical tags, which will be utilized for targeted modulation of dendritic cells (DCs) and T cells with immunomodulatory agents in my laboratory. We are also interested in exploring metabolic labeling, tracking, and targeting of stem cells.
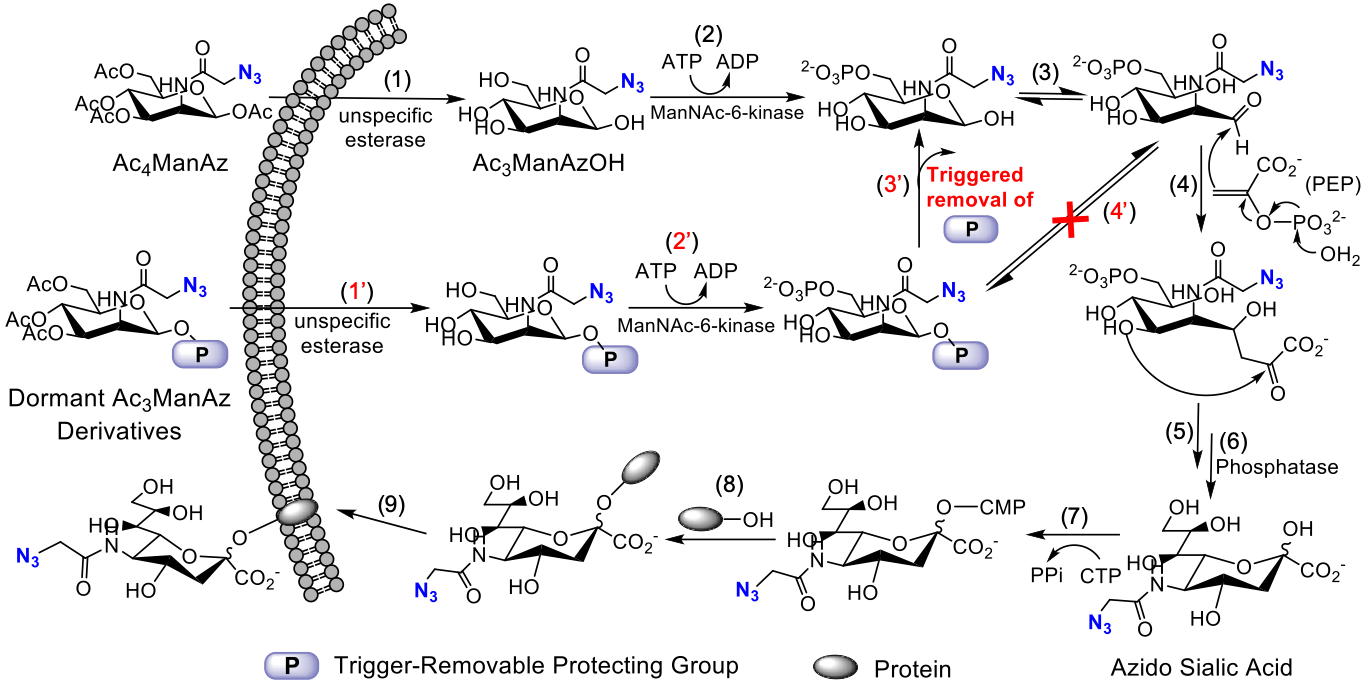
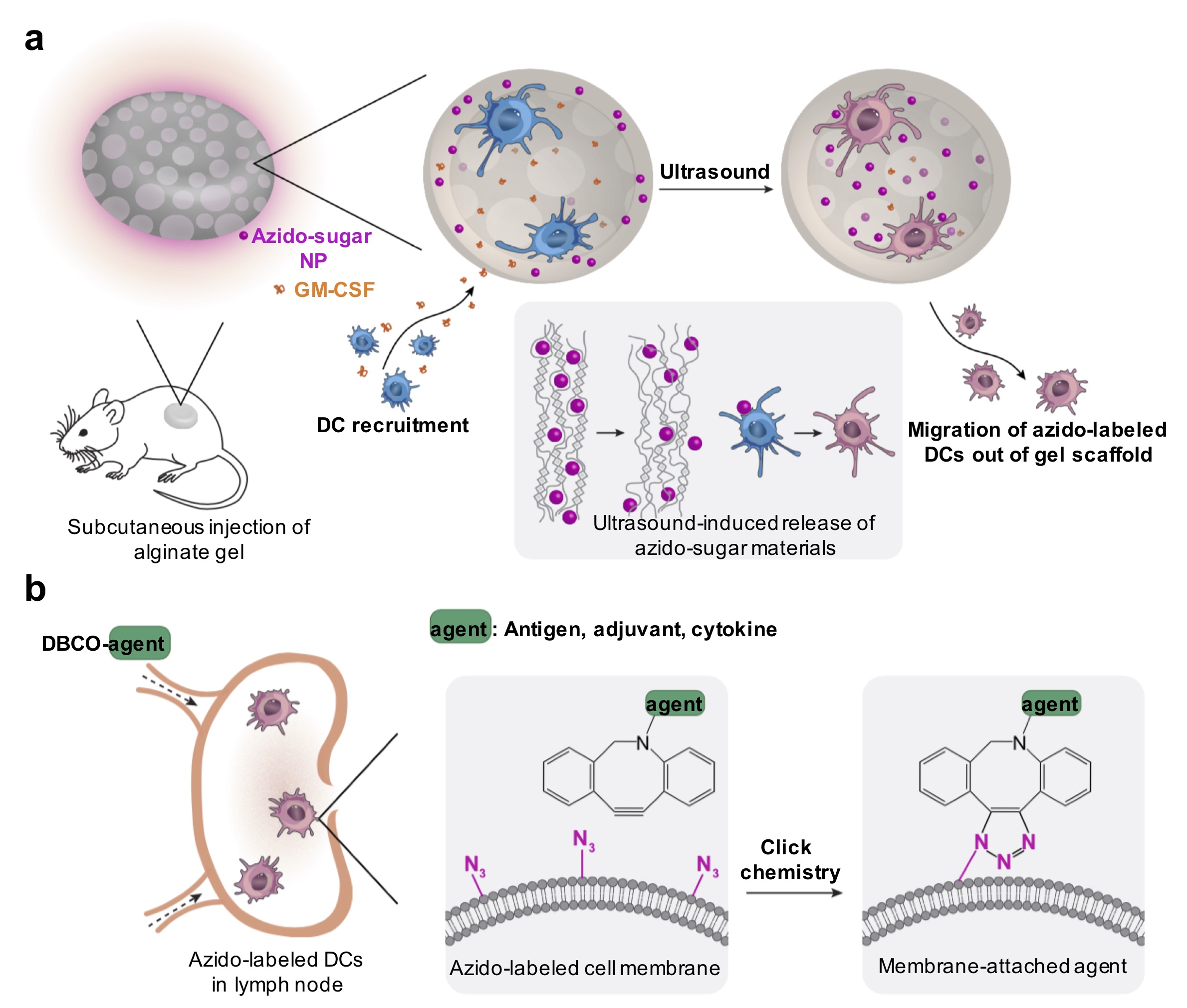
Representative publications:
(1) Wang H, Sobral M, Zhang D, Cartwright A, Li A, Dellacherie M, Tringides C, Koshy S, Wucherpfennig K, Mooney D. Metabolic Labeling and Targeted Modulation of Dendritic Cells. Nature Materials 2020, https://doi.org/10.1038/s41563-020-0680-1.
(2) Wang H, Wang R, Cai K, He H, Liu Y, Yen J, Wang Z, Xu M, Sun Y, Zhou X, Yin Q, Tang L, Dobrucka IT, Dobrucki LW, Chaney EJ, Boppart SA, Fan TM, Lezmi S, Chen X, Yin L, Cheng J. Selective In Vivo Cell Labeling Mediated Cancer Targeting. Nature Chemical Biology 2017, 13, 415-424.
(3) Wang H, Gauthier M, Kelly JR, Miller RJ, Xu M, O’Brien WD, Cheng J. Targeted Ultrasound Assisted Cancer-Selective Labeling and Imaging. Angewandte Chemie International Edition 2016, 55, 5452-5456.
2. Biomaterials-based Cancer Immunotherapy
Cancer immunotherapy has achieved significant progress in the past decade, but issues including off-target side effects and limited patient responses still exist. Biomaterial carriers of these therapies enable one to troubleshoot the delivery issues, amplify immunomodulatory effects, and home and manipulate immune cells in vivo. Our laboratory is particularly interested in developing biomaterials-based cancer vaccines and T cell therapies.
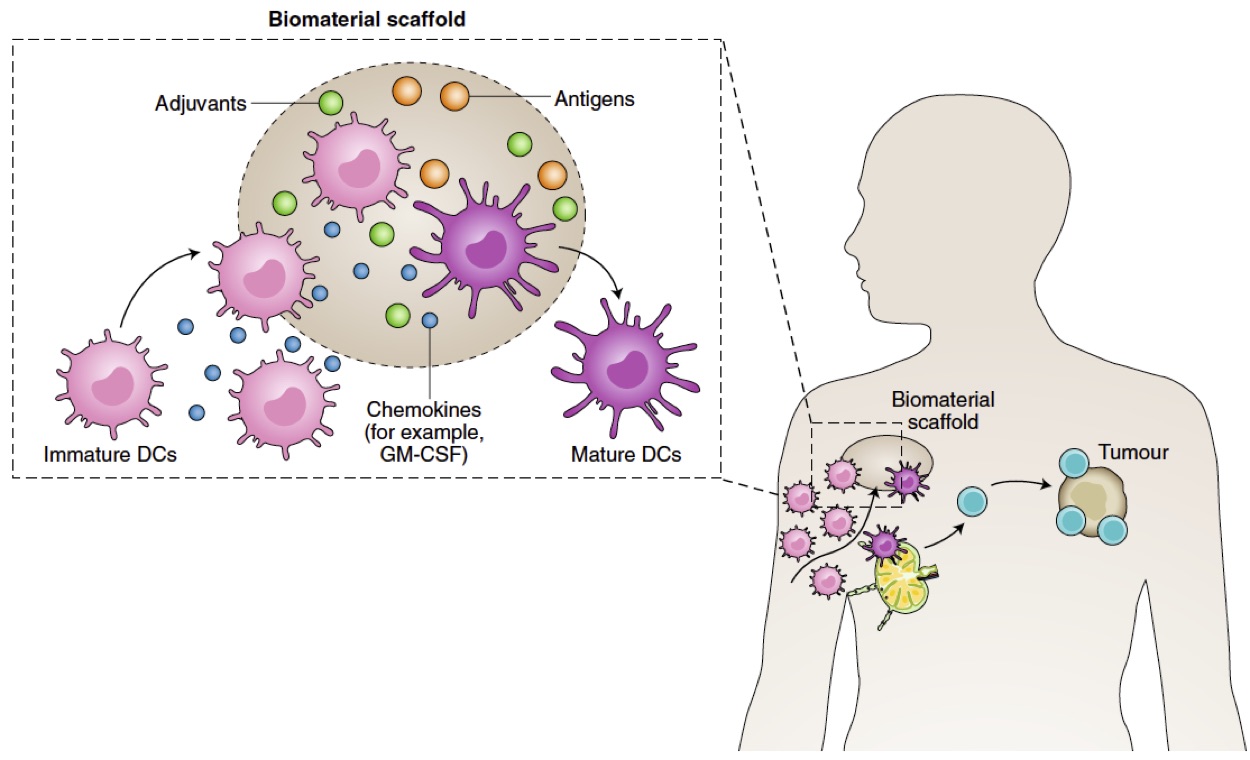
Representative publications:
(1) Wang H, Sobral M, Zhang D, Cartwright A, Li A, Dellacherie M, Tringides C, Koshy S, Wucherpfennig K, Mooney D. Metabolic Labeling and Targeted Modulation of Dendritic Cells. Nature Materials 2020, https://doi.org/10.1038/s41563-020-0680-1.
(2) Wang H, Mooney D. Biomaterial-Assisted Targeted Modulation of Immune Cells in Cancer Treatment. Nature Materials 2018, 17, 761-772.
3. Immunoengineering for Regenerative Medicine
Immune responses initiated upon tissue injury or biomaterial implantation are deemed critical for tissue restoration. Current efforts to regulate these immune responses towards facilitating tissue regeneration largely focus on magnification of M2-type macrophages and non-inflammatory cytokines. As key mediators of adaptive immunity, T cells and dendritic cells also play a critical role in regulating tissue regeneration processes. Our laboratory is interested in developing biomaterial approaches to modulate these immune cells in vivo to promote tissue restoration. We will also explore local, controlled release of immunomodulatory agents to facilitate bone and nerve repair.
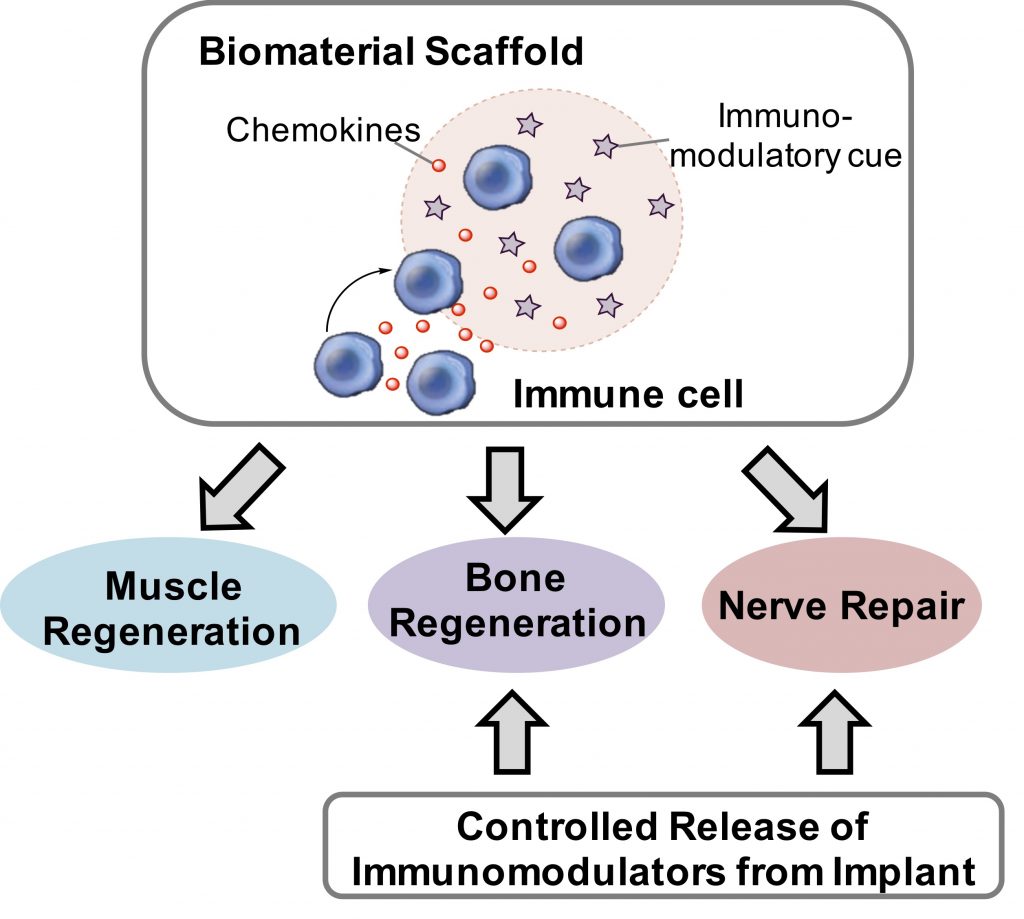
4. Nanoscale and Macroscale Biomaterials
Our laboratory will continue to develop advanced biomaterials including hydrogels, inorganic scaffolds, polymeric micelles, polymer-drug conjugates, and liposomes for applications of drug delivery, cell and tissue engineering, and in situ immunomodulation. One particular interest is the development and understanding of macroporous biomaterials that enable precise recruitment and modulation of immune cells in the context of cancers, regenerative medicines, and autoimmune disorders.
Representative publications:
(1) Wang H, Bo Y, Liu Y, Xu M, Cai K, Wang R, Cheng J. In Vivo Cancer Targeting via Glycopolyester Nanoparticle Mediated Metabolic Cell Labeling Followed by Click Reaction. Biomaterials 2019, 218, 119305.
(2) Wang H, Xu M, Xiong M, Cheng J. Reduction-Responsive Dithiomaleimide-Based Nanomedicine with High Drug Loading and FRET-Indicated Drug Release. Chemical Communications 2015, 51, 4807-4810.
(3) Wang H, Tang L, Tu C, Song Z, Yin Q, Yin L, Zhang Z, Cheng J. Redox-Responsive, Core-Cross-Linked Micelles Capable of On-Demand, Concurrent Drug Release and Structure Disassembly. Biomacromolecules 2013, 14, 3706-3712.