Chromatin, a complex of DNA and proteins in the eukaryotic cell nucleus governs various cellular processes including DNA replication, DNA repair and transcription. The main focus of the Prasanth Lab is to delineate how chromatin modulates these fundamental processes. Aberrant chromatin organization as well as chromatin deregulation are a leading cause of multitude of diseases. Epigenetic mechanisms play a pivotal role in regulating cellular processes and are essential for organismic development and cell fate and function.
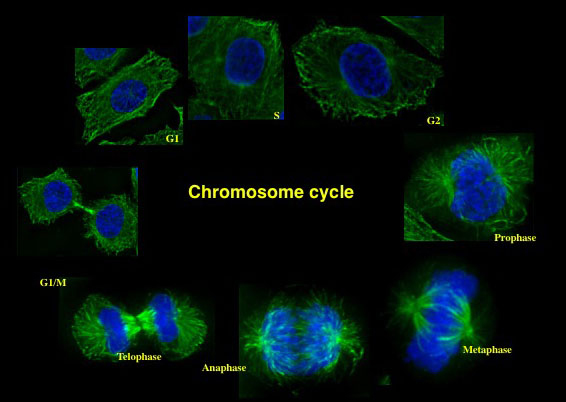
Linking DNA replication to chromatin organization:
Accurate duplication of genetic material and its faithful transmission to newly formed daughter cells is critical to maintain genome stability. ORC and other human replication initiation proteins have been shown to be required for replication of Epstein Barr virus (EBV) that is associated with Burkitt’s lymphoma. Other than their bonafide roles in DNA replication, ORC proteins are required for heterochromatin organization, centrosome copy number maintenance, accurate cell cycle progression and cell division. We are interested to understand how DNA replication and chromatin organization are coordinated in mammalian cells. In metazoans, ORC associates with origin DNA during G1 and with heterochromatin in post-replicated cells. However, what regulates the binding of ORC to chromatin is not understood.
ROLE OF ORCA IN DNA REPLICATION: We identified a novel, highly conserved, leucine-rich repeats and WD40 repeat do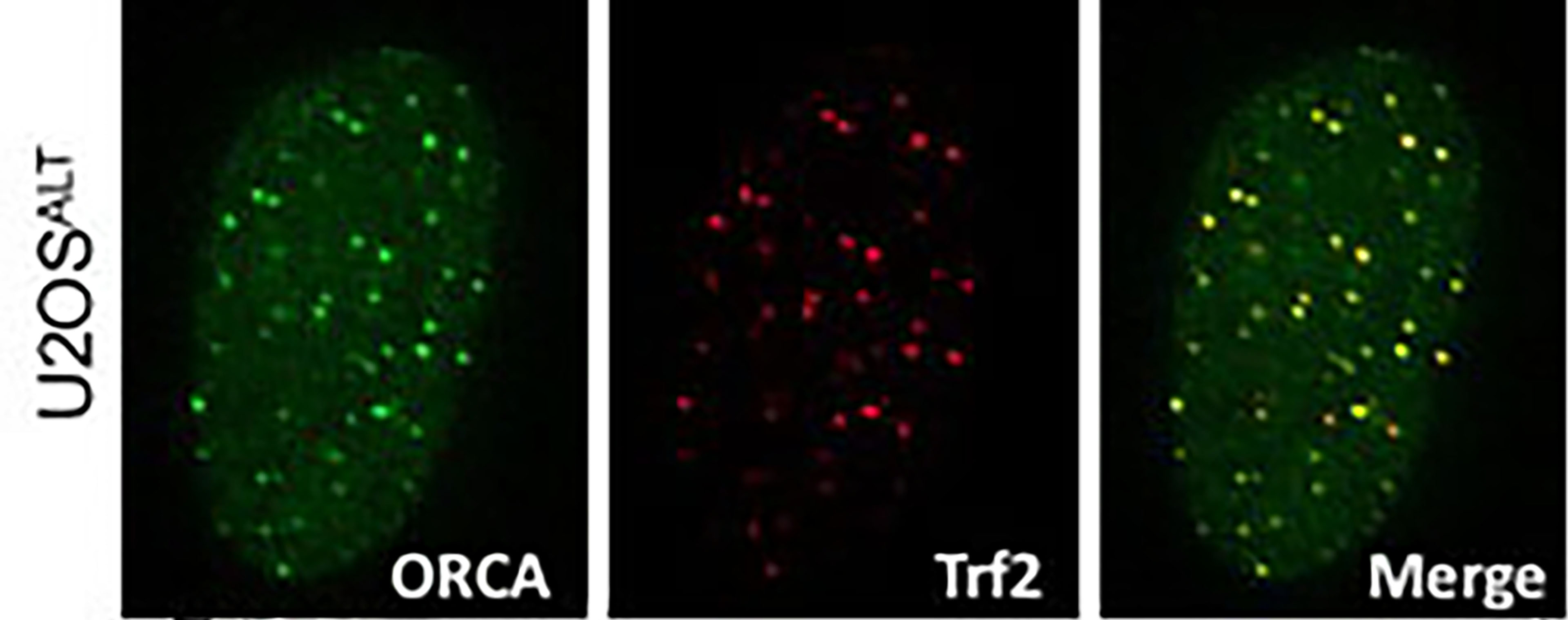 main-containing protein 1 (LRWD1) or ORC-associated (ORCA) in human cells that influences the ORC association to chromatin. ORCA and ORC associate with heterochromatic structures, including centromeres and telomeres and recognizes specific post-translational modifications on histones. Our studies focus on ORC and its regulation by ORCA to elucidate their roles in DNA replication, heterochromatin organization and mitosis.
main-containing protein 1 (LRWD1) or ORC-associated (ORCA) in human cells that influences the ORC association to chromatin. ORCA and ORC associate with heterochromatic structures, including centromeres and telomeres and recognizes specific post-translational modifications on histones. Our studies focus on ORC and its regulation by ORCA to elucidate their roles in DNA replication, heterochromatin organization and mitosis.
The goals of this project include:
- Role of ORCA in DNA replication initiation
- Role of ORCA at heterochromatin
- Regulation of ORCA during different stages of the cell cycle
This project is funded by NIH RO1.
SPATIO-TEMPORAL CONTROL OF DNA REPLICATION: In mammalian cells, chromosomal domains occupy a specific nuclear position and replicate at defined times during S-phase of the cell division cycle. The spatiotemporal pattern of DNA r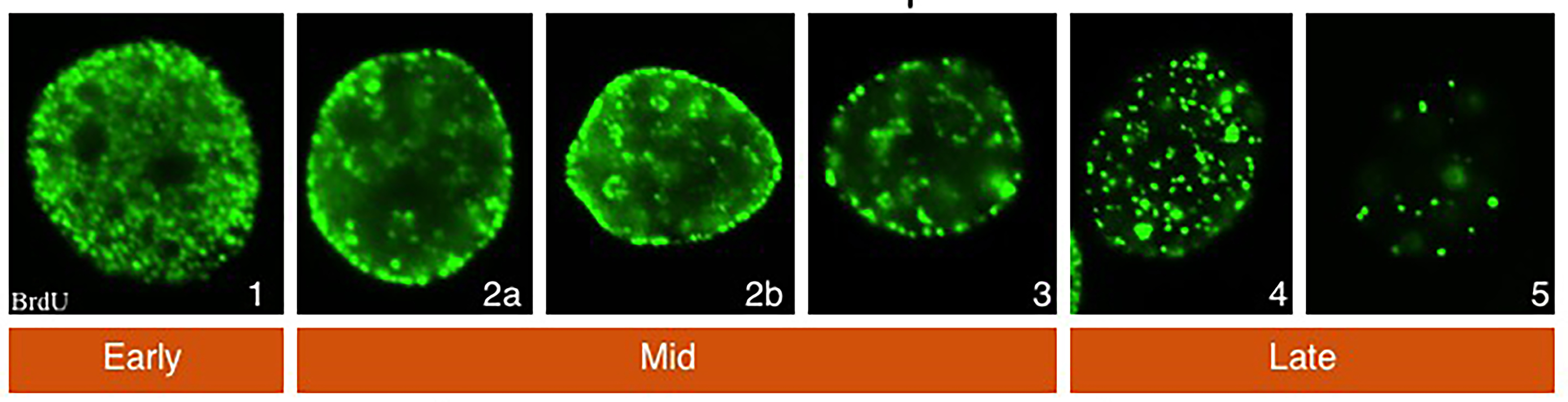 eplication within a given cell type is heritable and is established during G1. There are, however, few insights into the events and molecules that govern the spatiotemporal patterning of DNA replication. Our studies focus on determining how Orc1 contributes to and/or reads the epigenetic mark in G1 that determines the replication timing and patterning.
eplication within a given cell type is heritable and is established during G1. There are, however, few insights into the events and molecules that govern the spatiotemporal patterning of DNA replication. Our studies focus on determining how Orc1 contributes to and/or reads the epigenetic mark in G1 that determines the replication timing and patterning.
The goals of this project include:
- Role of ORC in regulating the spatio-temporal dynamics of DNA replication
- Role of ORC in regulating genomic stability
- Role of PTM on ORC in regulating the cell cycle
This project is funded by NSF.
Regulating gene expression through chromatin organization:
Accurate control of gene expression is crucial for cell survival and is clearly dependent on the chromatin status. In eukaryotes, higher order chromatin structure governs crucial cellular processes including DNA replication, transcription and post-transcriptional gene regulation. Specific chromatin-interacting proteins play vital roles in the maintenance of chromatin structure. Understanding the molecular network that together establishes heterochromatin structure and triggers transcription repression remains far from complete. We are interested to understand the relationship between transcription repression and chromatin organization in the eukaryotic genome. 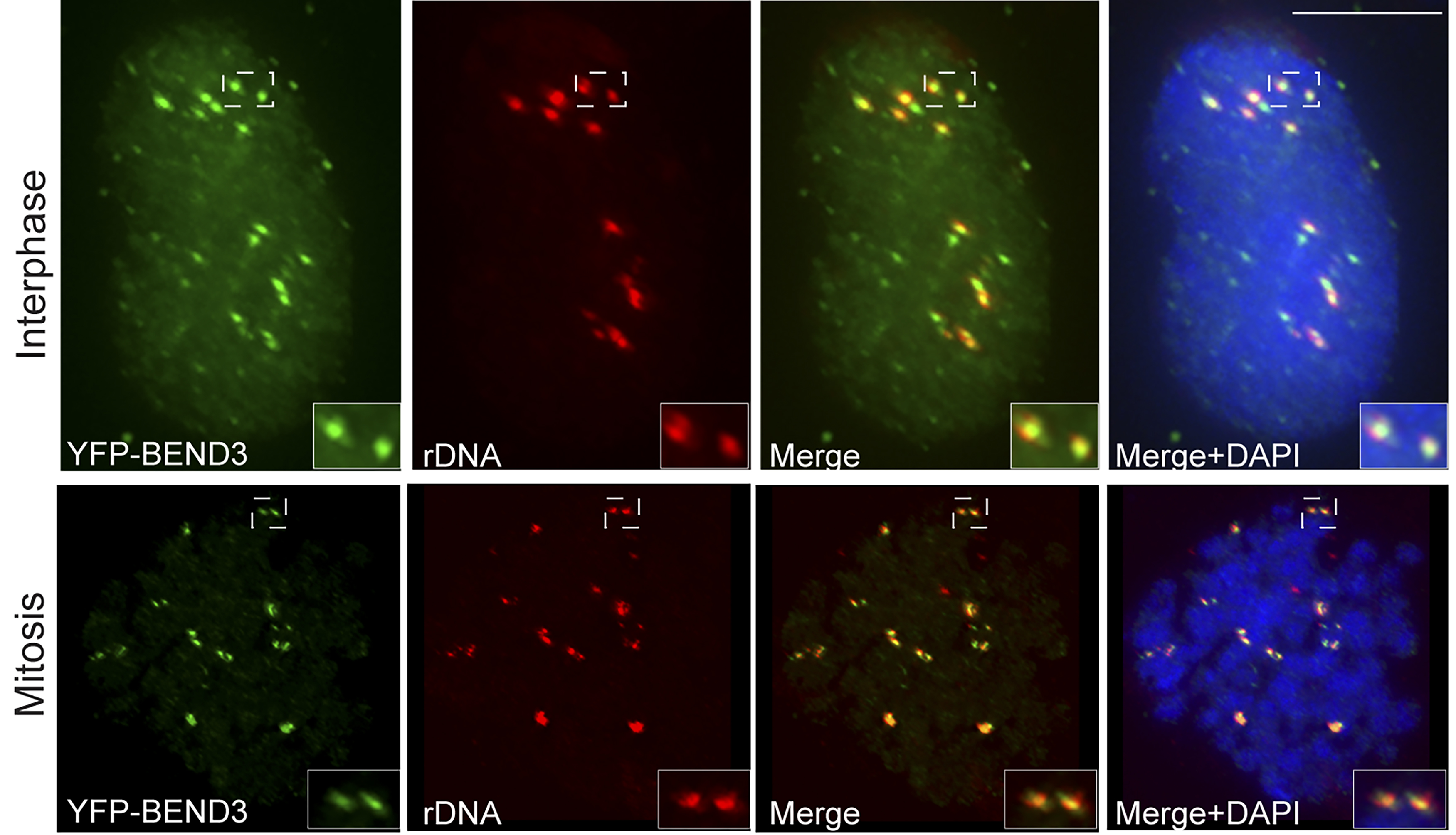
We have identified BEND3, a quadruple BEN domain containing protein that binds to heterochromatic sites and functions as a transcription repressor. We find that BEND3 is a rDNA transcription repressor that acts together with NoRC to actively coordinate the establishment of rDNA silencing. We seek to investigate the molecular mechanism of BEND3 action in mammalian cells.