Introduction: Read the following Learning Objectives, Overview, Purpose, Background, and Safety Considerations for transforming bacterial cells. Once you have read the information, follow this link to begin your adventure.
Learning Objectives: To learn how to introduce DNA into bacteria. Students will learn to transform plasmid DNA into bacteria and verify the presence of the plasmid by phenotype. They will then experimentally determine the copy number of a plasmid using qPCR in Lab 4.
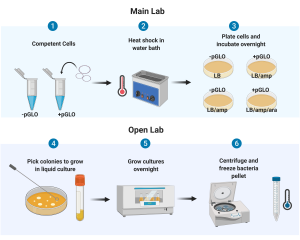
Overview: pGLO plasmid will be transformed into bacteria by heat shock. Transformed colonies will be “picked” and grown overnight in liquid culture. The OD of the expanded bacteria culture will be measured (to quantify number of bacteria cells) and the bacteria will be collected. pGLO plasmid will then be isolated from these transformed bacteria.
Purpose: Transformation of pGLO plasmid into E. coli allows the production of our gene of interest, GFP, in E. coli. Plating the transformed cells on different media (ex. +/- arabinose) verifies the plasmid features or “controls” on GFP expression. Once we select for transformed colonies of bacteria (those that have taken up the pGLO plasmid) we can then expand the number of cells by growing the colony in liquid culture (an “overnight” culture). By measuring the OD of the overnight culture, we will be able to use the calibration curve produced in Lab 2 to quantify the number of bacteria in the overnight culture. The overnight liquid culture can be centrifuged to collect the bacteria, which can then be frozen for later use.
Background: The introduction of DNA into bacteria, called transformation, is a widely used technique in recombinant DNA technology. Bacteria cells that are able to uptake DNA are called “competent”. Competence can either be a natural attribute (for example, in the bacteria Streptococcus pneumoniae) or can it be induced by chemical stressors. Bacteria can be made to be “chemically competent”, where they will uptake DNA in response to heat shock, or “electrocompetent”, where they will uptake DNA in response to an electrical pulse. In this lab we will be using heat shock to introduce plasmid DNA into E. coli DH5-α. DH5-α is a strain that is specifically engineered for use in lab work. They have been modified to have increased transformation efficiency and lack endonuclease activity.
Safety Considerations: E. coli strain DH5-α is a nonpathogenic strain of E. coli. It has been genetically modified to only grow on enriched medium. Standard lab practices must be followed including proper surface decontamination, hand washing, PPE, and waste disposal. These procedures will be reviewed at the start of the lab. Please ask your instructor/TA if you have any questions or concerns.
Ampicillin can cause allergic reactions or irritation to the eyes, respiratory system, and skin. If ampicillin contacts your eyes, use the eye wash station to rinse your eyes with water for a minimum of five minutes and report the incident to your instructor/TA for subsequent reporting.
Ultraviolet light will cause damage to skin and eyes. When using the UV pen light be sure to not direct the light at skin or eyes.