The diffuse and Stern layer of an ionic liquid on graphene electrodes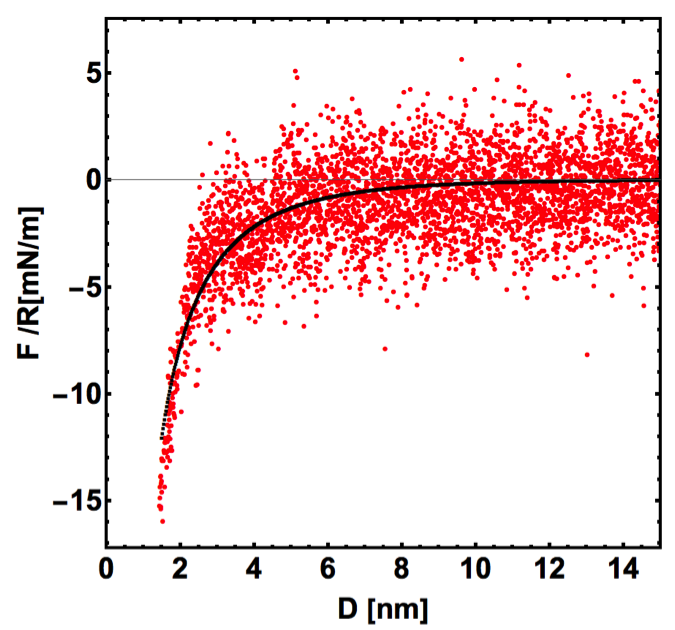
Graphene is a promising next-generation conducting material with the potential to replace traditional electrode materials in supercapacitors. Since energy storage in supercapacitors relies on the electrolyte-electrode interface, here we elucidate the interfacial subnanometer structure of a single component liquid composed solely of cations and anions – an ionic liquid- on electrified graphene. We study the effect of applied potential on the interaction between graphene and a silicon tip in an ionic liquid and describe it within the framework of the Derjaguin-Landau-Verwey-Overbeck (DLVO) theory. The energy is stored in an electrical double layer composed of an extended Stern layer, which consists of multiple ion layers over ~2 nanometers, beyond which a diffuse layer forms to compensate the applied potential on graphene. The electrical double layer significantly responds to the applied potential, and it shows the transition from overscreening to crowding of counterions at the interface at the highest applied potentials. It is proposed that surface charging occurs through the adsorption of the imidazolium cation to unbiased graphene (likely due to π-π interactions). This study scrutinizes the electrified graphene-ionic liquid interface, with implications not only in the field of energy storage, but also in lubrication.
Figure 1: Measured force-separation curve for bilayer graphene supported on gold in [EMIM][TFSI] at OCP and calculated by the DLVO theory . The fit gives the surface potential of bilayer graphene (0.196 V), the surface charge of the tip (-0.7 mC/m2), and the decay length (6 nm). Since non-DLVO forces are neglected, the fit to the DLVO theory is only performed at separations larger than 2 nm with a Hamaker constant= 1.1×10-20 J.
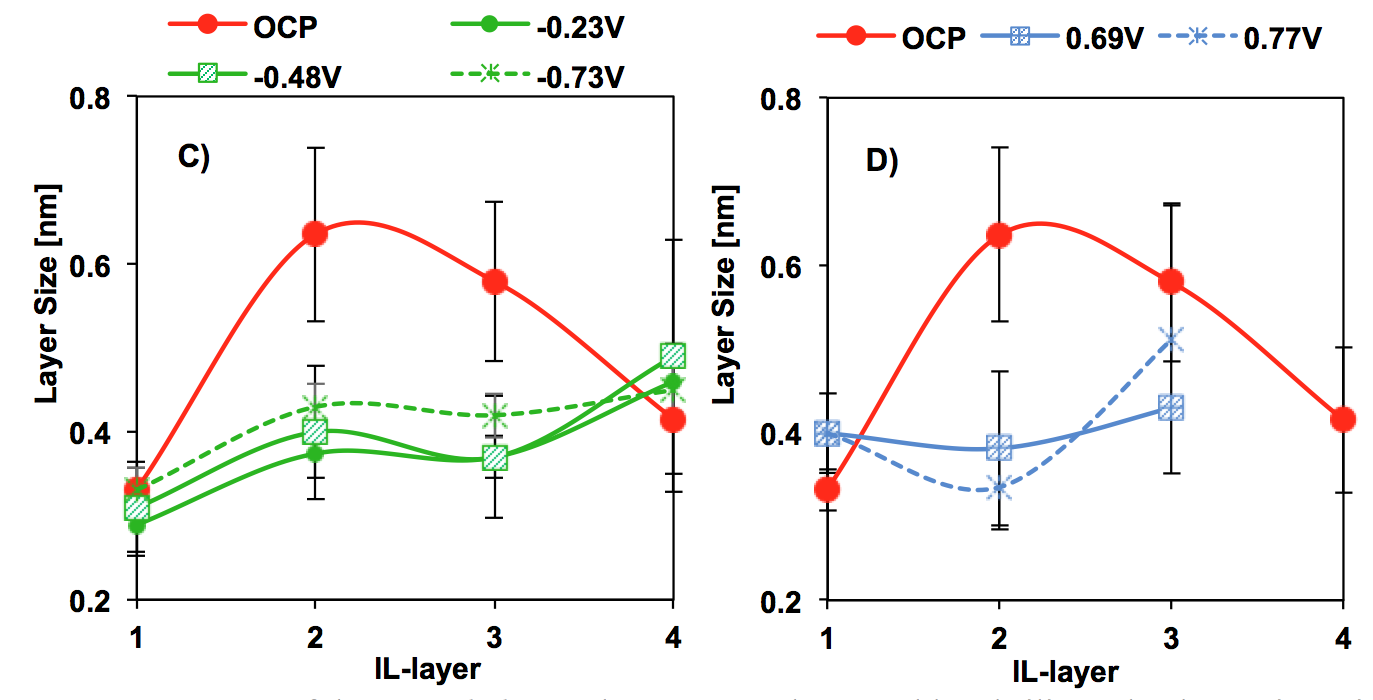 The concept of energy storage by a supercapacitor relies on the storage of charge in a thin EDL, which, according to the classical EDL theory, is expected to be sub-nanometer thickness for an electrolyte at high concentration, after the diffuse layer collapses. Although the electron distribution at the graphene electrode side can strongly influence the interfacial capacitance, it has been demonstrated that the capacitance is dominated by the EDL in the IL for more than four graphene sheets. In this case, the potential-dependent differential capacitance could be roughly estimated with the effective Debye length. Our results show that, for this particular IL, the effective thickness of the EDL on graphene is >1 nm and hence, larger than ideally expected for supercapacitors (<1 nm). Importantly, the measured change of the layer size distribution with potential shown in our force measurements supports that the compacity parameter (or packing efficiency) of [EMIM][TFSI] is not constant but it depends on the applied potential, which is not considered in the simplified EDL theory. The high configurational and conformational entropy of the IL ions, and the ion dissociation as a function of the potential are important parameters to consider for the selection of IL electrolytes for energy storage on graphene electrodes.
The concept of energy storage by a supercapacitor relies on the storage of charge in a thin EDL, which, according to the classical EDL theory, is expected to be sub-nanometer thickness for an electrolyte at high concentration, after the diffuse layer collapses. Although the electron distribution at the graphene electrode side can strongly influence the interfacial capacitance, it has been demonstrated that the capacitance is dominated by the EDL in the IL for more than four graphene sheets. In this case, the potential-dependent differential capacitance could be roughly estimated with the effective Debye length. Our results show that, for this particular IL, the effective thickness of the EDL on graphene is >1 nm and hence, larger than ideally expected for supercapacitors (<1 nm). Importantly, the measured change of the layer size distribution with potential shown in our force measurements supports that the compacity parameter (or packing efficiency) of [EMIM][TFSI] is not constant but it depends on the applied potential, which is not considered in the simplified EDL theory. The high configurational and conformational entropy of the IL ions, and the ion dissociation as a function of the potential are important parameters to consider for the selection of IL electrolytes for energy storage on graphene electrodes.
Figure 2: Structure of the extended Stern layer on gold-supported graphene. The data points give the mean value of the Gaussian distribution fitted to the layer thickness distribution and the variance is shown as an error bar. For bilayer graphene at OCP and C) negative and D) positive potentials.
Publications:
- L. Andres Jurado, Rosa M. Espinosa-Marzal, Insight into the Electrical Double Layer of an Ionic Liquid on Graphene, Scientific Reports 7, 2017.