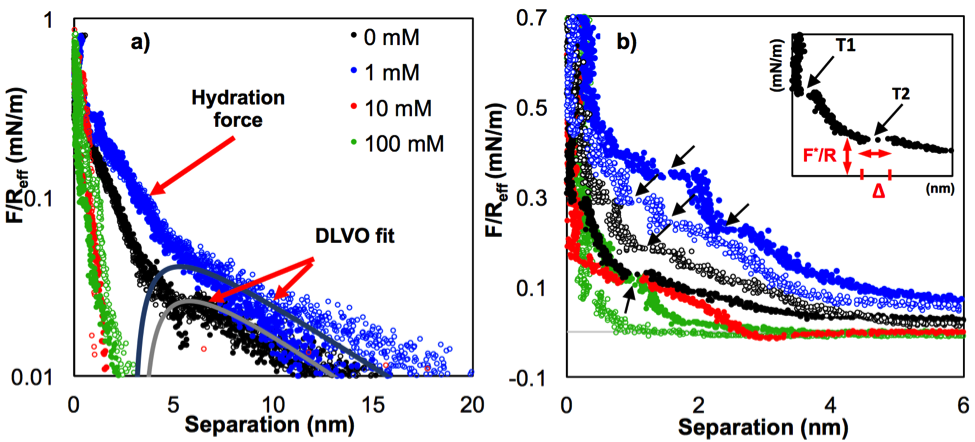
Figure 1: Representative force-separation curves between calcite and a silica colloid in CaCl2/CaCO3 solutions at separations a) smaller than 20 nm and b) smaller than 6 nm. The color scheme is consistent throughout this work, black for 0 mM, blue for 1 mM, red for 10 mM, and green for 100 mM CaCl2. Fits based on the DLVO theory lead to surface potentials for calcite, ψ_c=-12.8±2.2mV, -12.2±2.5 mV and -1.8±1.4 mV, and for silica, ψ_s=-6.3±0.9 mV, -7.6±2.3 mV and +57.8±12.4 mV, at 0 mM, 1 mM and 10 mM, respectively, and regulation parameters pc=0.62±0.07 and ps=0.88±0.07. The steps in the repulsive force (indicated by arrows in b) result from the squeezing of layers of water and hydrated ions. The inset in b) shows the FTT closer to the hard wall (T1), the subsequent FTT (T2) at a larger surface separation, the layer thickness (Δ) and the layering force (F*/Reff).
The electrical double layer of calcite (by Yijue Diao)
Because the SFA is limited to mica, colloidal probe atomic force microscopy was used to confine the calcite-water interface with a silica microsphere and to measure DLVO and non-DLVO forces between calcite and silica (Figure 1). Through the statistical analysis of the oscillatory component of the hydration force as a function of the calcium concentration, the subnanometer interfacial structure of atomically flat calcite is resolved in confinement in aqueous solution. A comparison with single calcite surface studies allowed us to elucidate the influence of nanoconfinement on the calcite-solution interface. By confining calcite, both layering species, water and hydrated calcium ions, are squeezed out (Figure 4b).
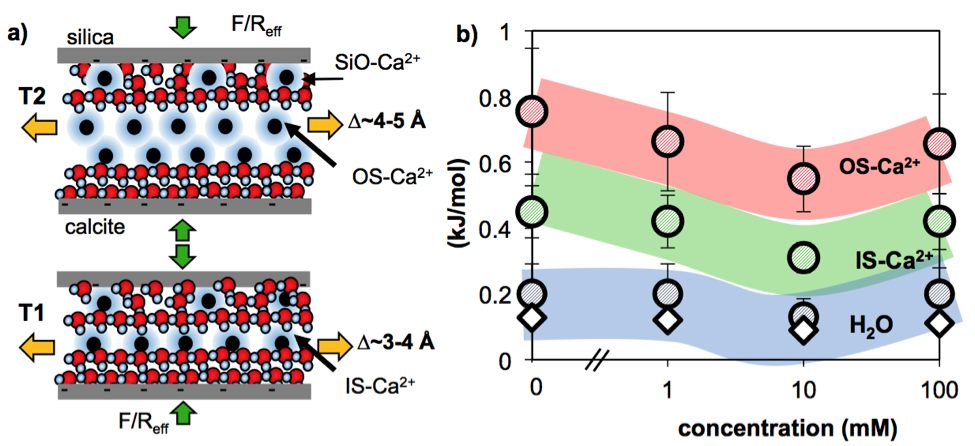
Figure 2: Energetics of squeezing-out a solution confined between calcite and silica upon applied load; (a) shows a cartoon for calcite-silica systems with a possible scenario, and b) shows the estimated work per mole applied to squeeze out OS-Ca2+, IS-Ca2+ ions and water molecules confined in calcite-silica systems (circles) and silica-silica systems (diamonds).
Figure 2 shows a cartoon of the confined solution between calcite and silica based on the present study. Similar to the unconfined calcite-solution interface, the confined solution is composed of layers of water and hydrated ions; in force measurements they are squeezed out upon an applied load, thereby leading to steps in the hydration force. The steps with a larger size (∆~3-5 Å) are detected at larger surface separations and are attributed to layers of hydrated calcium ions (IS-Ca2+ for ∆~3-4 Å and OS-Ca2+ for ∆~4-5 Å), followed by a smaller transition (∆~2.4-2.8 Å) that is closer to the size of water molecules. The collective shift to larger thicknesses indicates that larger species, i.e. more hydrated calcium ions relative to either less hydrated calcium ions or water molecules, populate the layers further away from the surfaces. In the example in Figure 5, layers of OS-Ca2+ (top) and IS-Ca2+ (bottom) ions are resolved. Importantly, the population of IS-Ca2+ ions appears to be more significant in confinement than on unconfined calcite, perhaps due to the forced-dehydration by the applied load.
Publications:
- Y Diao, RM Espinosa-Marzal, Molecular insight into the nanoconfined calcite–solution interface. Proceedings of the National Academy of Sciences 113 (43), 12047-12052, 2016.
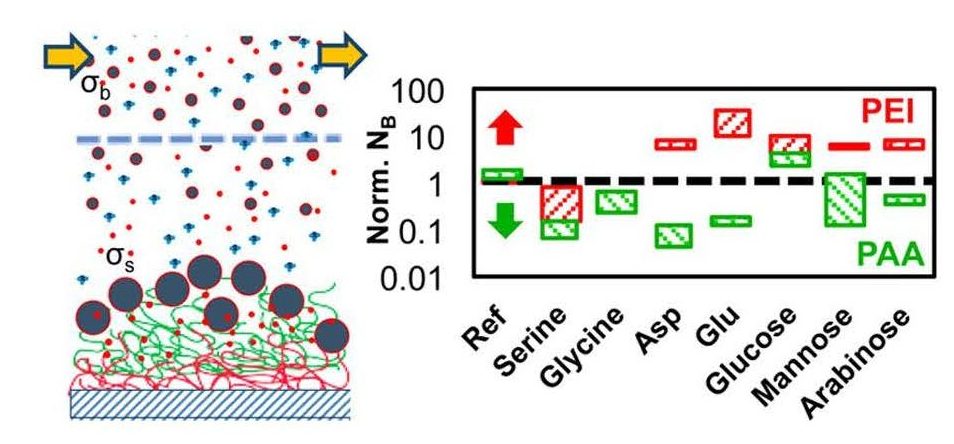
Figure 3: Mineralization on PEI- and PAA-polymer films in the presence of selected amino acids and monosaccharides (NB is the nuclei number).
Chemical interfacial processes during biomineralization (by Josue Lopez)
Calcium carbonate is one of the most common minerals in nature. Many marine organisms, for example, have complex organic matrices that can tune the mineralization of calcium carbonate to form highly functional structures (e.g. shells, spicules, etc.). These organic-inorganic interactions have also been proposed to stabilize the amorphous phase of calcium carbonate (ACC), previously believed to be transient. ACC’s mechanical properties are of great interest due to its enhanced toughness and lack of fracture planes. To better understand the mechanisms that underlie biomineralization, studies on the multiple scales through which calcium carbonate interacts with its environment have been performed:
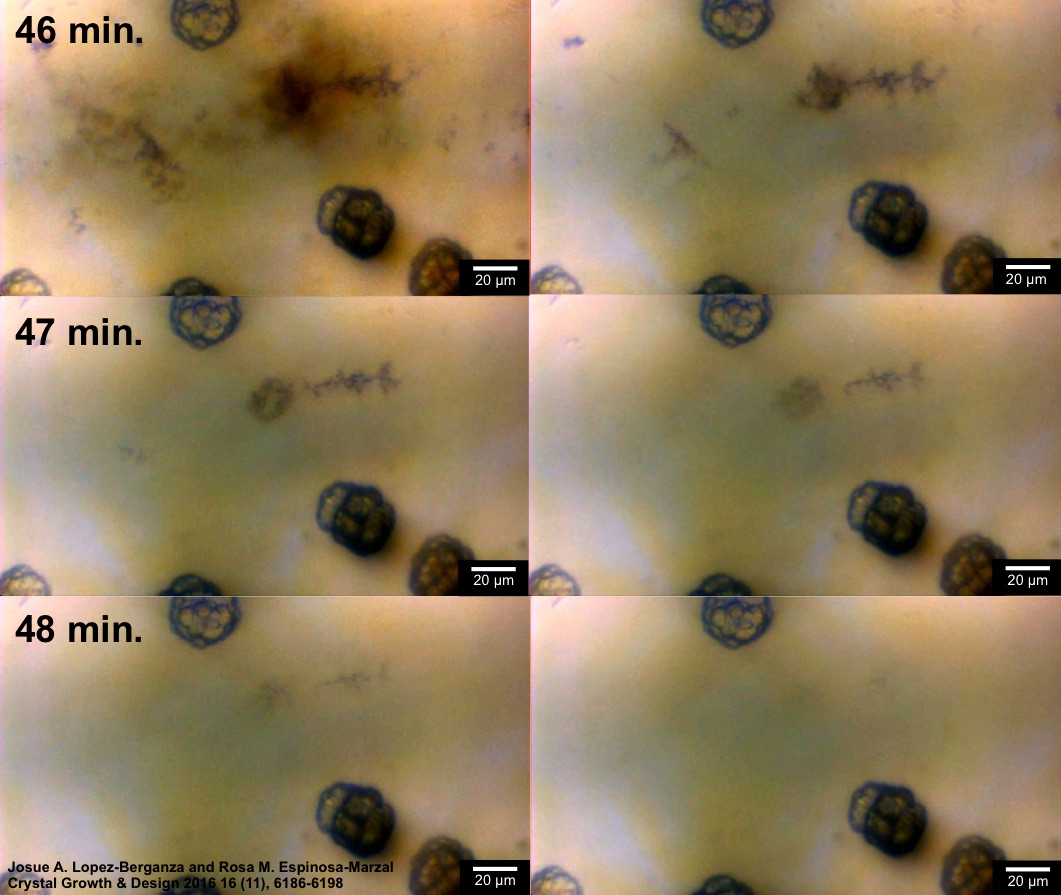
Polymers and soluble additives can mediate the nucleation and growth of different types calcium carbonate crystals. The influence of two chemically distinct surfaces and the presence of various amino acids and polysaccharides on the kinetics of calcium carbonate growth were monitored in situ through absorbance measureme
nts and light microscopy . Parameters derived from classical nucleation and crystal growth models were used to quantify the competitive and synergistic effects that the organic additives exert on the growing crystals in a biomimetic flow cell (Figure 3). Through these studies, we provide new avenues to exploit the organic-inorganic interactions present in nature to aid the development of smart biomimetic composite materials. This work is funded by the National Science Foundation (CMMI-14-35920).
At the molecular level, ab-initio simulations were used to characterize the structural and energetic properties of calcium carbonate’s hydration shell. This “layer” of water molecules that exists during the initial stages of calcium carbonate formation and is believed to play an important role in the pathway to biomineral growth.
Publications:
- Josue Lopez, Yijue Diao, Sudhakar Pamidighantam, Rosa M. Espinosa-Marzal, Ab Initio Studies of Calcium Carbonate Hydration, The Journal of Physical Chemistry A 119 (47), 11591-11600.
- J.A. Lopez-Berganza, RM Espinosa-Marzal, Mechanistic approach to predict the combined effects of additives and surface templates on calcium carbonate mineralization, Crystal Growth & Design 16 (11), 6186-6198.