Ongoing Research Projects in OpNTech
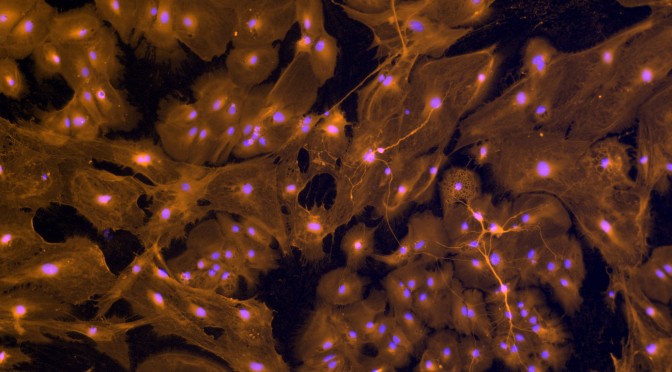
- OptoMEA for measuring signaling efficiency of neuronal networks: Optogenetics has opened up new avenues for exploring neuronal functions using non-invasive stimulation of specific neurons. We have recently established an investigative tool – OptoMEA – that combines optogenetic stimulation of channelrhodopsin-2 expressing neuronal networks with long-term electrophysiological measurement from multiple locations of the network using a multi-electrode array (MEA) dish. This combinatorial approach is being used to induce, detect and track short- and long-term changes in spontaneous and evoked neuronal network activity in response to optical stimuli. In parallel, multi-parametric assays for measuring signaling efficiency of a neuronal network are being developed. The long-term goal for this project is to develop, test and validate this assay by connecting it to the “health” of a neuronal network under normal and emulated diseased conditions. We are currently testing this assay with an in vitro model of neuronal inflammation and neurodegeneration.
- Development of new tools for early detection of Alzheimer’s disease: Alzheimer’s disease is the most common form of dementia. An estimated 5.2 million Americans of all ages have Alzheimer’s disease in 2013. It is the sixth leading cause of death in the United States. Unfortunately, no standalone definitive diagnostic test for AD exists today. It is recognized that a test from early diagnosis before onset of cognitive decline and deposition of Aβ plaques in the brain will substantially improve outcomes of available treatments, allow patients and caregivers time to plan efficiently for the future healthcare needs which will eventually reduce costs for effective management of the disease. In this project we are developing two tools for AD diagnosis, both based on detection of Aβ oligomers. Our hypothesis is that as the plaques are formed over decades through seeded-oligomerization of soluble Aβ monomers, the equilibrium concentrations of Aβ oligomers are expected to be elevated for AD patients compared to age-matched healthy individuals. Additionally, as an increase in Aβ oligomer concentration needs to precede plaque formation, Aβ oligomers can be used as a biomarker for early detection of AD long before onset of cognitive decline. The first assay uses double-probe fluorescence correlation spectroscopy for detection of Aβ monomer to Aβ oligomers ratio from body fluid. The second one employs a multi-electrode array based neurochip for electrical recording from hippocampal neurons exposed to cerebrospinal fluid samples emulating normal and diseased conditions of the brain. Recent results show that very low concentrations of Aβ oligomers transiently change the firing pattern of neurons without killing these cells, opening up new possibilities for diagnosis (or monitoring of treatment response) using this neurochip device.
- Neuronal network synchronization, plasticity and effects of neurotrophins: Synchronized bursting of electrical activity is found in many brain areas and has also been implicated in the pathophysiology of neuropsychiatric disorders such as epilepsy, Parkinson’s disease, and schizophrenia. Despite extensive studies of network burst synchronization, how this type of network wide synchronization can be reduced, abolished or enhanced, and how elevated levels of neurotrophins modify network synchronization events are insufficiently understood. In this project, we have combine electrical recording using multi-electrode array with optical stimulation of cultured channelrhodopsin-2 expressing cortical neurons to study and manipulate network burst synchronization. Additionally, we are investigating effects of brain derived neurotrophic factor, a neurotrophin involved in development and plasticity of brain networks, on the synchronization of these networks.
- Development of an all optical stimulation and recording system for neuronal activity: Spatially resolved optical recording of spontaneous or evoked changes in membrane voltage from neurons (action potentials and field potentials) has been one of the biggest challenges of neuroscience research. Optogenetic stimulation, as described above, allows non-invasive and selective stimulation of neurons using light. Similarly, recording of neuronal electrophysiology by detecting light from neurons ─ or optical electrophysiology ─ potentially will permit an all optical recording device enabling electrophysiological recording without an invasive contact electrode. To this end, we are integrating a calcium imaging module based on genetically encoded calcium sensors (GCaMP6s and RCaMP), and a fast single action potential imaging module based on Archaerhodopsin 3 (Arch) to the optogenetic-electrophysiology setup (described above), for an all optical measurement of brain functions from neuronal networks. Once developed and operational, this multi-modal detection platform will enable all optical stimulation and recording from neuronal networks without use of any invasive electrode contacts.
- Quantitative study of mechanical perturbation to neurons in a petri dish using HAMr device: Traumatic brain injuries (TBI) are common and very costly in medical care and lost productivity each year. The majority of brain injuries that occur each year are classified as mild TBI (mTBI). One of the most common cases of mTBI is concussion, and the long-term effect of repeated concussions is still unclear. The aim of this project is to investigate the relationship between repeated mechanical impacts to brain networks and the resulting level of impact-induced inflammation. A custom-designed mechanical impactor – HAMr device – is used at OpNTech. We have coupled this device to living neuronal networks cultured in a dish, and have successfully used this device for measuring if there is a safe exposure limit to mechanical force that does not cause any permanent neurological damages to this network. Additionally, we are studying if modulation of temperature post-exposure is neuro-protective. Post-impact changes in the neuronal networks are currently tracked by measuring expression levels of anti-inflammatory proteins using advanced single cell fluorescence imaging technologies. In parallel, we are also investigating immediate changes in network activity post-impact using single cell calcium imaging of GCaMP6s expressing neurons.
- Development of optogenetic muscle-powered 3D-printed biological machines (collaboration with Dr. Rashid Bashir, University of Illinois): Bio-integrated soft robots, or “bio-bots”, can accomplish such objectives of robotics as sensing, storage and processing of signals, and a resultant response such as actuation. Such biological machines can target a myriad array of applications that require robotic function in response to complex sets of dynamic environmental signals. The most intuitive demonstration of a biological machine is one that can generate force and produce motion. Dr. Bashir’s laboratory has previously developed a millimeter-scale bio-bot in which muscle cells seeded in a synthetic extracellular matrix applied traction forces to compact into a strip capable of contractility and force generation in response to external electrical signals. In this project, Dr. Bashir, Dr. Roger Kamm (M.I.T.) and their students have joined forces with Dr. Sengupta to test functions of optogenetic bio-bots constructed using light-active skeletal muscle cells. In OpNTech, we are measuring light-induced movements of an engineered contractile muscle ring that can be used as a modular biological “building block”. Most recent results show that these muscle rings can be used to build a range of locomotive bio-bots that can steer and walk across 2D surfaces in response to external optical signals.
- Effects of brain derived neurotrophic factor on signaling pathways of satiation (collaboration with Dr. Steven M. Simasko, Washington State University): Nutrient induced release of cholecystokinin (CCK) from the small intestine acts to facilitate efficient gastrointestinal function and promotes the process of satiation. The peripheral effects of CCK are specifically mediated by CCK1 receptors (CCK1Rs). The first aim of this collaborative project is to decipher the intracellular signal transduction pathways for CCK signaling. The second aim is to investigate how brain derived neurotrophic factor affects this signaling of satiation. A compelling body of evidence has emerged that indicates a pivotal role of BDNF in the development of processes that normally regulate food intake, satiation and control of body weight. For example, when BDNF is knocked out in select central nervous system areas, mice become hyperphagic and obese. In collaboration with Dr. Steven. M. Simasko, we are studying the relationship between altered vagal signaling in the absence of BDNF at the cellular level to connect disrupted meal patterns in knockout animals to compromised satiation. For this project, we are using long- and short-term primary cultures of vagal neurons isolated from nodose ganglia of rats to measure evoked response to CCK under different conditions using single-cell calcium imaging. The results suggest that while BDNF is not necessary for neurite extension, its absence causes a large increase in number of CCK-sensitive nodose neurons thereby altering sensitivity towards satiation-related signaling in vagal afferent neurons. In the future, we hope to link these research findings directly to obesity and an in vitro obesity model will be created.
- Development of a fiber-bundle based all optical stimulation and imaging platform (collaboration with Drs. Stephen A. Boppart and Justin Rhodes, University of Illinois): Optogenetics is a rapidly developing field with an ever-expanding toolkit of molecular biology techniques to enable light-activated switching and control of cells, most commonly neurons. Equally significant advances have occurred in optical science and engineering. By understanding and exploiting physics-based principles of how light interacts in photonic crystal fibers (PCFs) and within imaging fiber bundles, it is possible to generate, control, and optimize a wide range of new optical parameters for in vivo optogenetic stimulation. Traditionally in in vivo optogenetic applications, light has been sent down single multi-mode optical fibers to diffusely illuminate the brain, relying on the molecular biology of optogenetically-modified neurons for cell and circuit specificity. This BRAIN EAGER project funding is developing and demonstrating the use of imaging fiber bundles, and the generation of specific light pulse parameters to enable spatially-resolved optogenetic stimulation and imaging of neural circuits in vivo. In the future, these novel neurotechnologies will enable new investigations underlying behavior and cognition in animal models, and one study to investigate the role of new hippocampal neurons in learning and behavior will be tested.