Waterborne pathogens sicken and kill people, especially young children in developing countries. According to the World Health Organization, diarrhea kills one person every minute worldwide . 90% of these deaths are children under the age of 5 in developing countries. Diarrhea is caused by waterborne pathogens, such as Vibrio cholerae, Cryptosporidium parvum oocysts, and rotavirus. Among waterborne virus, rotavirus is the most common pathogenic viruses detected in domestic wastewater and contaminated surface water. According to the Center for disease control and prevention, intestinal complication due to Rotavirus infection cause death of 600,000 children worlwide and hospitalization for 55,000 children in the US. Even though vacine against rotavirus infection has been available recently, rotavirus vaccination is effective in countries with high GDP, but much less effective in developing countries. Sustainable access to safe drinking water and adequate sanitation is the number one requirement for a healthy and productive society worldwide. Scientific knowledge is urgently needed to guide sustainable practice
Control of pathogenic viruses for water supplies include not only disinfection for drinking water treatment but also prevention of viral contamination due to untreated wastewater. We are doing a comprehensive study on the role of virus interfacial behavior in aquatic environments to gain fundamental understanding of factors influencing the fate and transport of virus in subsurface environment.
Bacteriophage MS2 and Rotavirus Adsorption to Hematite Nanoparticles: Role of NOM and Divalent Cations
Student researcher: Leo Gutierrez (MS in 2007)
Collaborators: Professor Jim Economy and his student Xuan Li in Material Science.
We have found that even though hematite nanoparticles with diameters from 5 to 20 nm provide an attractive substrate for the viruses, fouling by natural organic matter substantially reduces virus removal capacity. In addition, the presence of water hardness (i.e., Ca2+ and Mg2+) significantly increases virus adsorption because the divalent cations allow virus to adsorb on the NOM, which adsorbed on the surface of hematite nanoparticles. We also obtained microscopic evidence that adsorption of MS2 to hematite nanoparticles does not destroy the viral capsid. With appropriate solution conditions, we were able to desorb 40% of adsorbed viruses.
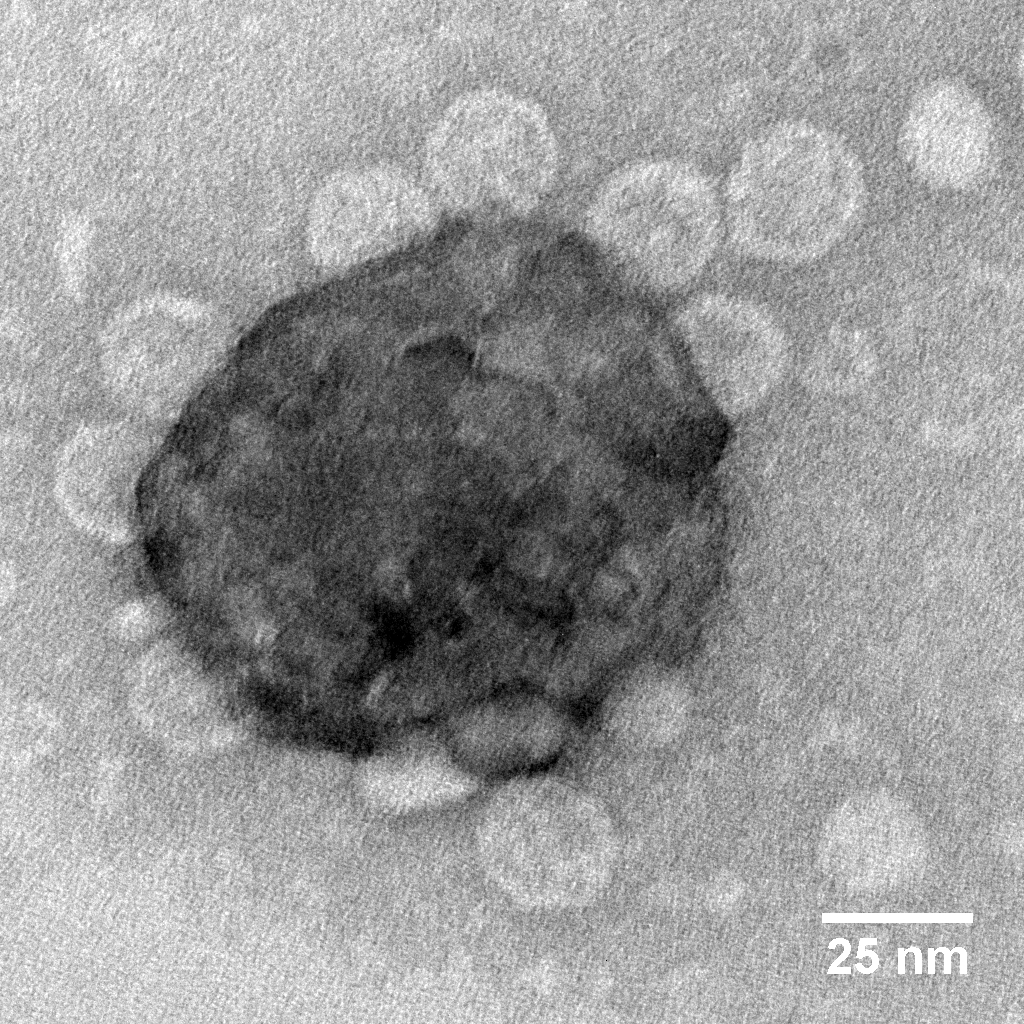 |
|
MS2 adsorb to hematite nanoparticles. Figure from Gutierrez et al., 2009. |
Interactions of Bacteriophage MS2 and Rotavirus with Surfaces Coated with Natural Organic Matter: Role of Solution Compositions
Researchers: Dr Baoling Yuan and Mai Pham (MS in 2009).
We started with using MS2 as a model virus because MS2 has been used as surrogate for disinfection and transport study. We proceed further with rotavirus. We use a quartz crystal microbalance (QCM) coupled with a radial stagnation point flow (RSPF) cell to study deposition kinetics of bacteriophage MS2 and rotavirus on silica surfaces coated with Suwannee River natural organic matter (SRNOM). We found that MS2 deposition rates increased gradually and stablized at an ionic strength of 60 mM. In addition, the maximum measured attachment efficiency is only 0.2, suggesting the role of steric repulsion. Experimental data suggest that electrostatic and steric repulsion were the two main deposition
mechanisms of MS2 on either silica surfaces or silica surfacees coated with SRNOM. This low value of alpha measured for MS2 on silica and SRNOM-coated surfaces suggest that the interactions of MS2 with environmental surfaces are weak, and MS2 are expected to be highly mobile.
In addition, we also found that MS2 remain monodispersed if we purified MS2 and stored them in bicarbonate buffer or 1 mM NaCl solution. Phosphate buffer causes MS2 to aggregate.
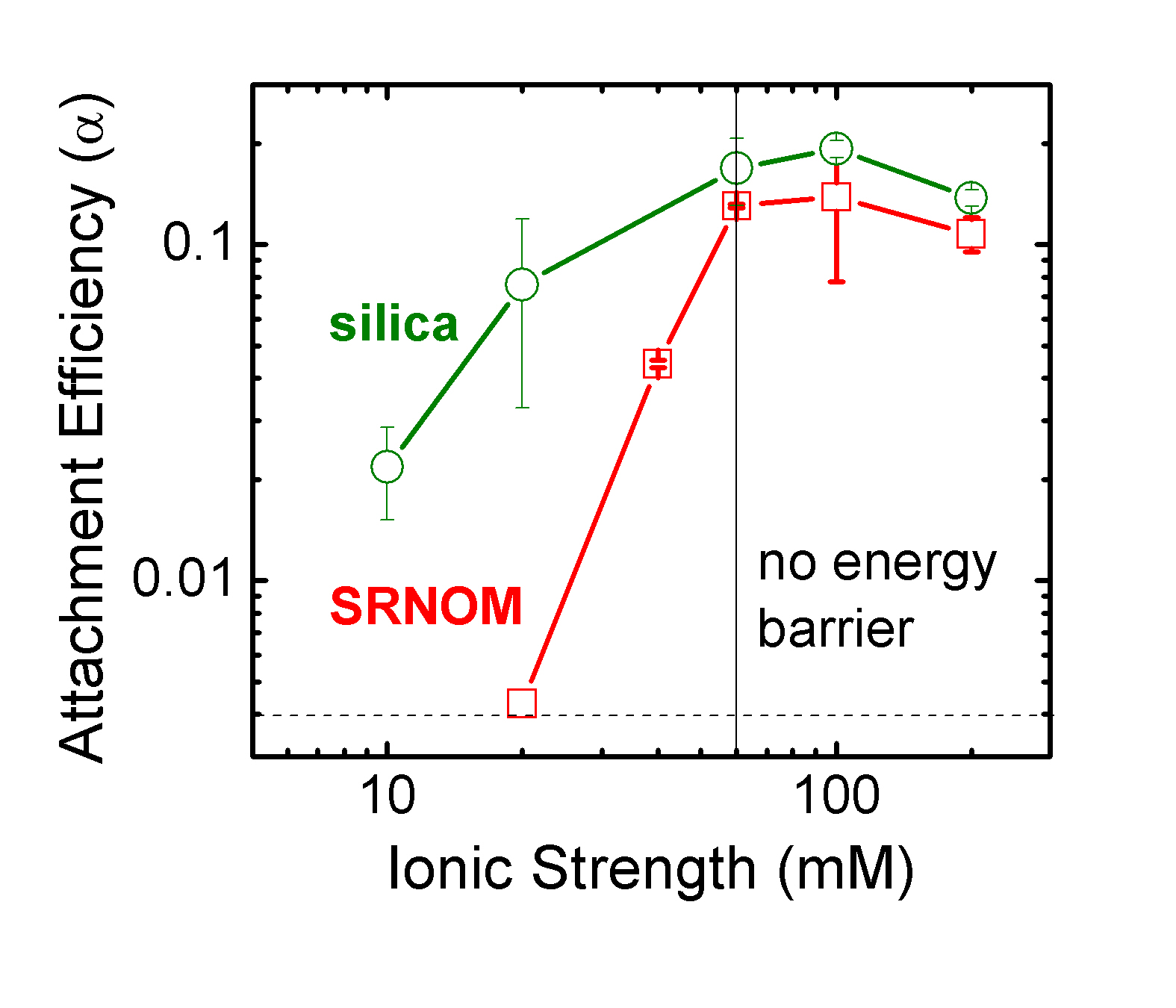 |
Deposition rates of MS2 on bare and SRNOM-coated silica in a QCMD radial stagnation point flow cell. Figure from Yuan et al., 2008. |
We further found that attachment efficiencies of MS2 onto the SRNOM surface were seven to seventeen times higher in the presence of Ca2+ than in the presence of Mg2+. A similar trend was
observed for the adsorption of polyglutamic acid, which is one of the carboxylate-containing amino acid residues found on the surface of MS2 capsids present on SRNOM-coated surfaces. The difference in attachment rates in a solution containing either Ca2+ or Mg2+ can be explained by a stronger tendency of Ca2+ compared to Mg2+ to form cation bridges by binding to carboxylate groups of both the SRNOM and the MS2 capsids. Moreover, higher attachment efficiencies of MS2 onto the SRNOM-coated silica surfaces compared to those on bare silica surfaces in the presence of either Ca2+ or Mg2+ at concentrations higher than 0.3 mM emphasized the important role of SRNOM carboxylate groups. Experimental data also showed reduced attachment efficiency of MS2 to SRNOM-coated surfaces in solution containing 1 mg/L SRNOM.
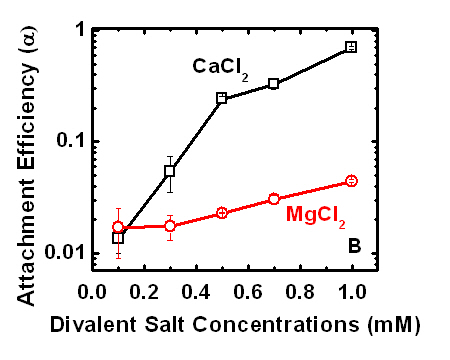 |
Deposition rates of MS2 on SRNOM-coated silica in a QCMD radial stagnation point flow cell. Figure from Pham et al., 2009. |
Aggregation of Bacteriophage MS2 and Rotavirus
Collaborators: Professor Steve Mylon (Department of Chemistry,
Lafayette College).
Student researcher: Leo Gutierrez (PhD in 2013)
Viruses like the bacteriophage MS2 have adopted similar strategies for stability against aggregation. In natural systems dissolved organic matter can adsorb to and effectively chang virus surfaces affecting the fate and transport of these viruses. We used time resolved dynamic light scattering to measure the aggregation kinetics of a model virus, the bacteriophage MS2, across a range of solution chemistries in order to understand what factors might destabilize viruses in aquatic systems. In monovalent electrolytes (LiCl, NaCl, KCl) aggregation of MS2 could not be induced within a reasonable kinetic timeframe, and MS2 was stable even at concentrations greater than 1.0 M. Aggregation of MS2 could be induced in divalent electrolytes when we employed Ca2+. This trend was also observed in solutions containing 10 mg/L Suwannee River organic matter (SROM) reference material. Even concentrations of Ca2+ as high 200 mM, diffusion controlled aggregation was never achieved demonstrating an additional barrier to aggregation. These results were confirmed by small angle X-ray scattering experiments, which indicate a transition from repulsive to attractive interactions between MS2 virus particles as monovalent salts are replaced by divalent salts.
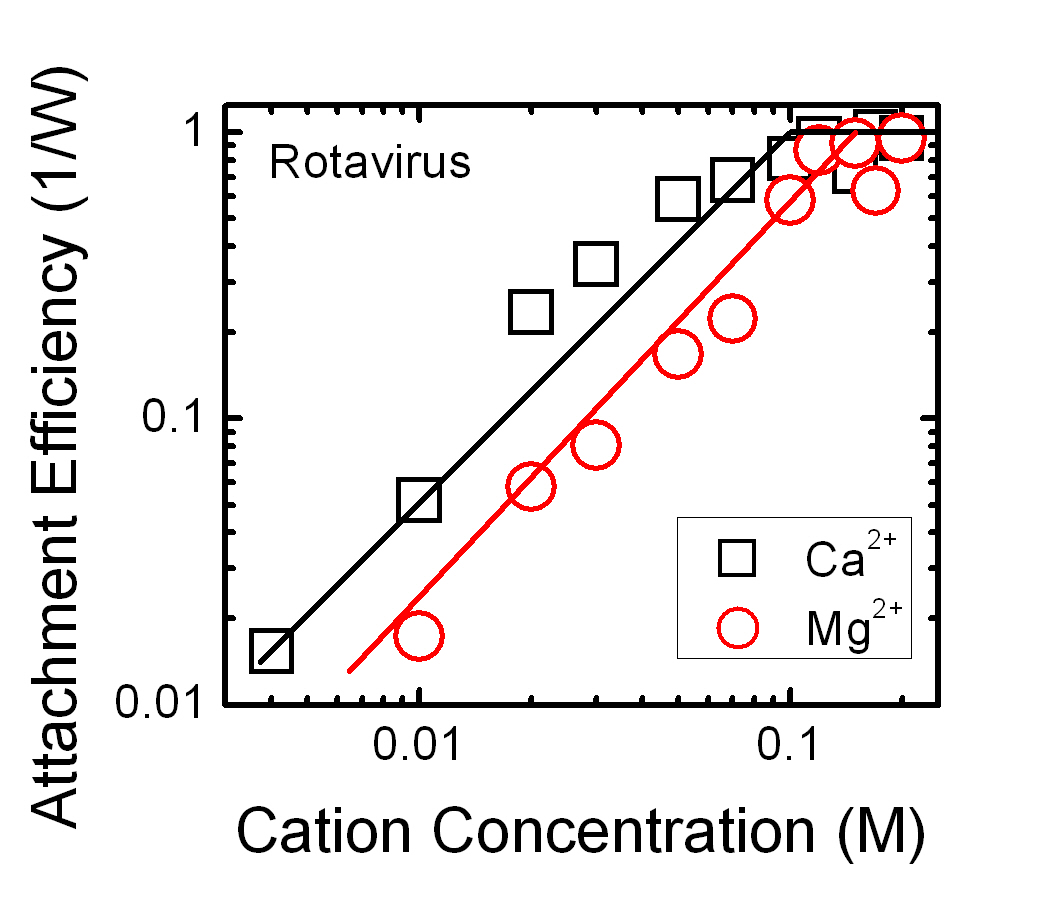 |
Attachment efficiency (1/W) of rotavirus in the presence of divalent cations. Aggregation rates for rotavirus were measured at various cation concentrations ranging from 4 mM to 200 mM in Ca2+ and 10 mM to 200 mM in Mg2+ and normalized as attachment efficiency with respect to the fastest measured aggregation rate (the lines are meant to delineate the regions between favorable and unfavorable aggregation). Figure from Mylon et al., 2010. |
Publication
- Wang*, H., Narihiro, T., Straub, A., Pugh, C., Tamaki, H., Moor, J. Bradley, I., Kamagata, Y., Liu, W.T, Nguyen, T.H., MS2 Bacteriophage Reduction and Microbial Communities in Biosand Filters, Environmental Science & Technology, 48 (12), pp 6702–6709, 2014, full text.
- Phan, A.D., Tracy, D.A., Nguyen, T.L.H., Viet, N.A, Nguyen, T.H., Electric potential profile of a spherical soft particle with a charged core, The Journal of chemical physics, 2013, Vol. 139, p. 244908, full text.
- Gutierrez*, L. and T.H. Nguyen, Interactions between rotavirus and natural organic matter isolates of different physicochemical characteristics. Langmuir, 2013, Vol. 29, pp 14460–14468, full text.
- Gutierrez*, L., and Nguyen, T.H. , Interactions between Rotavirus and Suwannee River Organic Matter: Aggregation, Deposition, and Adhesion Force Measurement, Environmental Science & Technology, 46 (16), pp 8705–8713, 2012, full text.
- Nguyen, T.H., Easter* N., Gutierrez* L., Huyett, L., Defnet, E., Mylon, S.E., Ferri, J., Viet, N.A., The RNA Core Weakly Influences the Interactions of the Bacteriophage MS2 at Key Environmental Interfaces, Soft Matter, 2011, Vol. 7, p. 10449-10456, full text.
- Bradley* I, Straub* A., Maraccini* P., Markazi S., Nguyen T.H., Iron Oxide Amended Biosand Filters for Virus Removal, Water Research, 45 (15), 4501-4510, 2011, full text.
- Gutierrez, L., Mylon, S.E., Nash, B., and Nguyen, T.H., Deposition and aggregation kinetics of rotavirus in divalent cation solutions, Environmental Science & Technology, 2010, 44, 4552–4557, full text.
- Brady-Estevez, A. S., Nguyen, T. H. , Gutierrez, L*.; Elimelech, M., Influence of Solution Chemistry on Viral Removal by a Single-Walled Carbon Nanotube Filter, Water Research, 2010, 44, 3773-3780, , full text.
- Mylon S., Rinciog C. Schmidt N., Gutierrez* L.; Wong G. and Nguyen, T.H. , Influence of Salts and Natural Organic Matter on the Stability of Bacteriophage MS2, Langmuir, 2010, 26 (2), 1035–1042, full text.
- Gutierrez* L., Li X., Wang J., Nangmenyi G., Economy J., Kuhlenschmidt T.B., Kuhlenschmidt M.S., and Nguyen, T.H. , Adsorption Of Rotavirus And Bacteriophage MS2 Using Glass Fiber Coated With Hematite Nanoparticles, Water Research, 2009, Vol. 43, p. 5198-5208, full text
- Pham* M., Mintz E.A., and Nguyen, T.H. , Deposition Kinetics of Bacteriophage MS2 to Natural Organic Matter: Role of divalent cations, Journal of Colloid and Interface Science, 2009, Vol. 338, Pages 1-9, full text.
- Yuan* B., Pham* M., and Nguyen, T.H. , Deposition kinetics of bacteriophage MS2 on a silica surface coated with natural organic matter in a radial stagnation point flow cell, Environmental Science and Technology, 2008, Vol. 42, p. 7628-7633, full text.
