Pathogens including Cryptosporidium parvum oocysts found in surface runoff are one of the leading causes of impaired river and estuary water. One hundred million cattle are able to produce approximately 600 oocysts annually for every person in the U.S. This number of oocysts far exceeds the infectious dose for humans. Knowledge on the fate and transport of C. parvum oocysts in agricultural runoff is currently lacking and is urgently needed to protect water supplies for many parts of the country. The results of this project will provide a scientific basis for water resources and environmental sustainability.
This project uses a multiscale approach to identify chemical and physical factors that influence attachment and mobility of C. parvum oocysts. A comprehensive understanding of these factors will be used to develop a model to predict the fate and transport of oocysts in the sub-surface environment. The objectives of this project are: (1) to investigate the role of oocyst wall macromolecules in the deposition and transport of C. parvum oocysts by systematically modifying the oocyst walls; (2) to determine the attachment mechanisms of C. parvum oocysts on inorganic (i.e. quartz) and organic (i.e. coated with natural organic matter) soil surfaces on a microscopic scale; and (3) to determine the transport of C. parvum oocysts in sub-surface environment in micromodel and column setups. The experimental approach ranges from a microscopic to a macroscopic scale. A novel microscopic technique consisting of a radial stagnation point flow (RSPF) cell combined with a microscope will be used to monitor attachment and detachment kinetics of oocysts under well-defined flow conditions in real time. Deposition and detachment experiments will be conducted with systematically varied solution conditions to determine the mechanisms of oocyst interaction with representative soil surfaces. Pore scale transport of oocyst will be studied using a precisely fabricated micromodel. Knowledge on oocyst deposition and transport at microscale will be applied to elucidate transport at continuum scale of column setup.
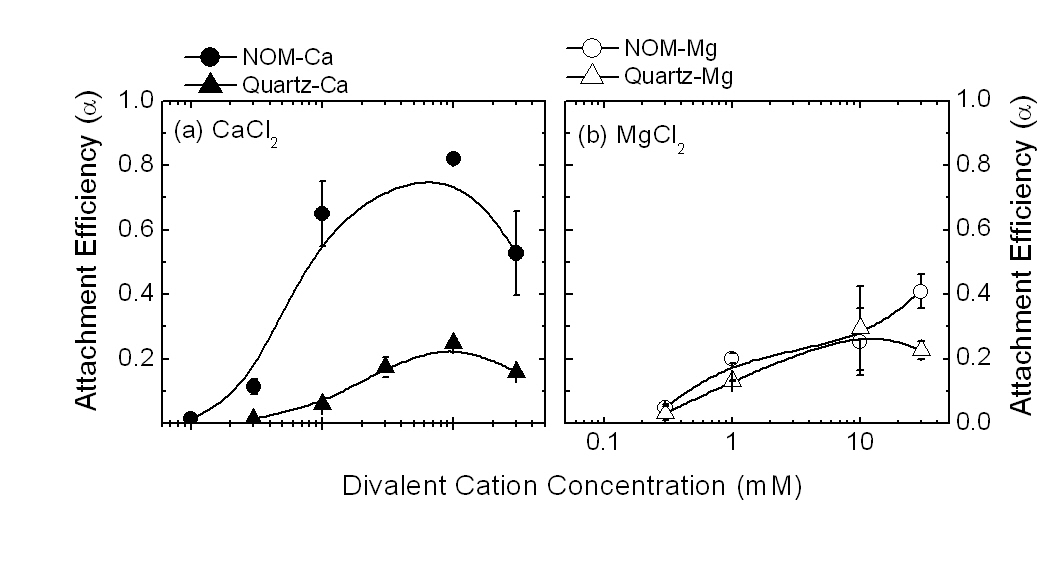
The main hypothesis of this project is that reversibility of oocyst deposition and subsequent transport depends on the composition of pore-water. Water contaminated with manure has significantly higher salt and organic matter than clean water such as rain. A significant portion of oocysts deposited on soil surfaces oocysts in solutions containing 3-30 mM ionic strengths is expected to detach during a sudden drop in ionic strength due to rain fall. Mobilization of the previously attached oocysts provides an additional rapid transport pathway of pathogens to water source. This hypothesis will be tested using both deposition experiments in a RSPF and transport experiments in a micromodel.
 |
||||
|
Microscopic observation of C. parvum oocyst deposition on the SRNOM-coated surface in secondary minima at 10 mM ionic strength. The image shown is a composite image generated by superimposing successive pictures taken over 20 min. Pictures were taken every second over the course of 30 min. Not all pictures were superimposed for clarity. C. parvum oocysts, which we traced, deposited onto the SRNOM surface and appear as single dark points marked with numbers. The paths of traced C. parvum oocysts are shown as light aligned points linked to the C. parvum oocysts marked with numbers. C. parvum oocyst 1 deposited with relatively higher velocity with an average velocity of 15.38 ?m/s (it traveled 76.9 um within 5 s); the dark point on its pathway is an oocyst that had already deposited on the SRNOM surface before C. parvum oocyst 1 appeared in the view area. C. parvum oocyst 2 was deposited 10 s after it appeared in the view area (traveling a distance of 90.1 um), released after 30 s, and deposited again 6 s later (traveling a distance of 12.5 um); eventually it was washed away by the radial flow after 95 s. C. parvum oocyst 3 was deposited 14 s after it appeared in the view area (traveling a distance of 97.4 ?m), released after 93 s, and eventually deposited 3 s later (traveling a distance of 10.2 um). C. parvum oocyst 4 deposited with a relatively lower velocity with an average velocity of 2.16 um/s (it traveled 30.2 um within 14 s). Experiments were carried out at ambient pH (around pH 5.6-5.8) and a temperature of 25 °C. Figure from Liu et al., 2009.
We fabricated a micromodel, which has 2-dimensional (2-D) microscopic pore structures consisting of an array of cylindrical collectors. This micromodel was used for real time monitoring of oocyst transport and attached oocyst distributions in transversal and longitudinal directions. In the micromodel, oocysts attached to the forward portion of clean collectors, where the flow velocity was lowest. After initial attachment, oocysts attached onto already attached oocysts. As a result, the collectors ripened and the region available for flow was reduced.
Publication and Presentations
|