Sayan Mitra
Sayan Mitra
Department of Electrical and Computer Engineering
University of Illinois at Urbana-Champaign
mitras@illinois.edu
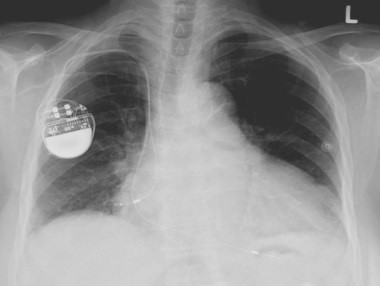
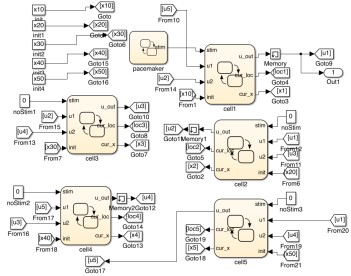
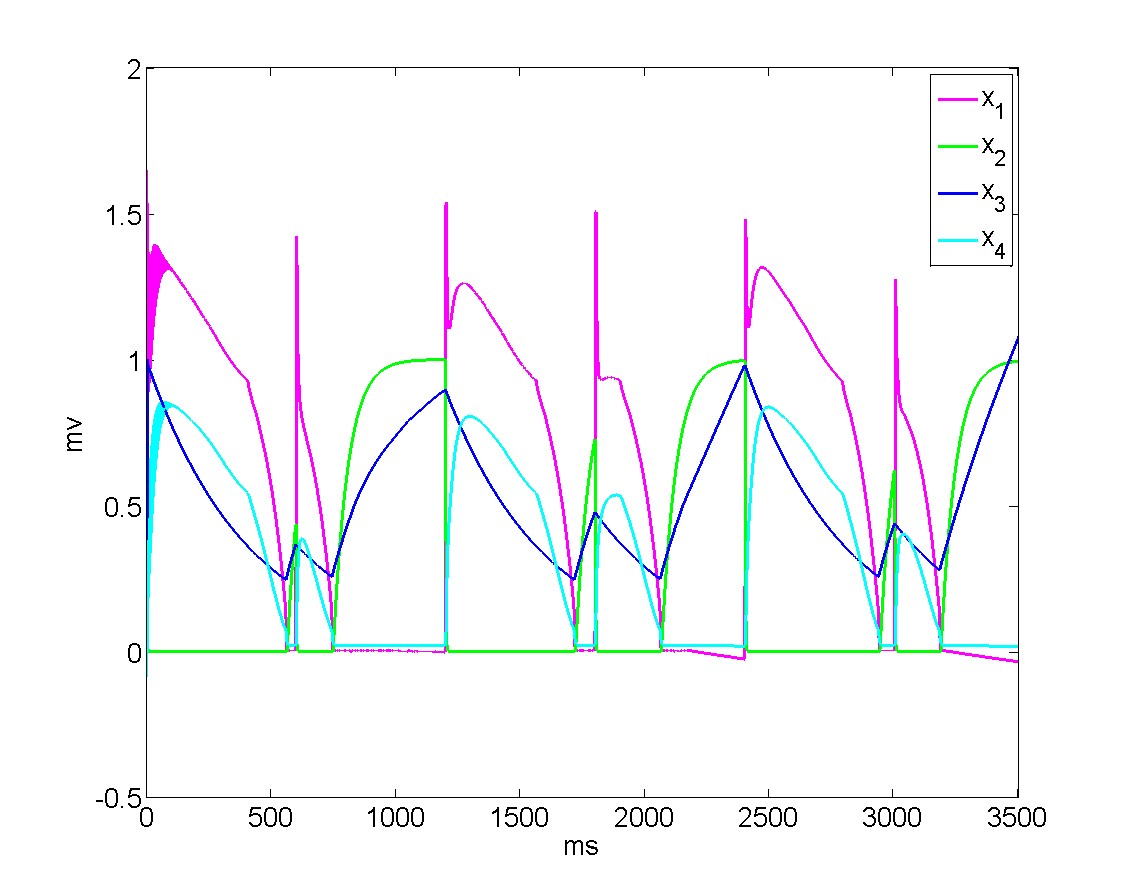
Millions of medical devices are recalled every year, and by some estimates, a quarter of these recalls are because of software and design errors. Broad discussions are taking place in industry, in federal agencies (especially the FDA), and in the research community on how to revamp the certification process for medical devices and processes. One of the approaches to achieving that goal is to use model-based design and certification for medical systems. That involves creating a model of the artifact, including its software as well as the physical process in which it is embedded, and then using techniques from control theory and software verification to find possible design defects or ascertain that there are none. We are developing tools that support model-based design and analysis. Recently, we showed that these tools can be used to analyze models of cardiac cell networks and their interactions with pacemakers to determine whether the device can drive the system into physiologically undesirable states. These technologies and tools can radically improve the development and certification processes for critical medical devices.
Sayan Mitra graduated from MIT in 2007 and spent one year as a postdoctoral researcher in the Center for Mathematics of Information at Caltech. His research has been recognized with several best paper awards, a National Science Foundation CAREER award (2011), an AFOSR Young Investigator Research Award (2012), and an IEEE-HKN Outstanding Teacher Award (2013). His research is aimed at developing algorithmic techniques for analyzing (and finding defects in) complex software systems that interact with physical processes.