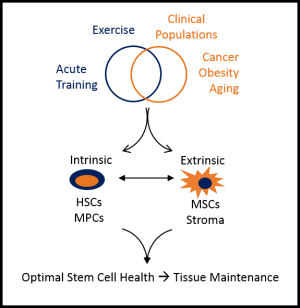
Research Overview
Degenerative conditions associated with aging, obesity and long-term effects of cancer treatment result in loss of tissue function due to decreased regenerative potential of endogenous tissue stem cells. Work in the Exercise and Stem Cell Physiology (ESC) Lab focuses on the optimization of tissue-resident adult stem cell populations by exercise. Maintaining the quantity, function and microenvironment of adult stem cells by exercise would allow for a type of “endogenous cell therapy” that would delay or reverse tissue dysfunction in chronic pathological conditions. Our approach uses the highly translatable and feasible intervention of regular exercise that has already been shown to be safe and effective in many chronic conditions. Our long-term vision is to be a world leader in endogenous cell therapy research by pioneering exercise-based interventions that optimize endogenous stem cell function. To achieve this goal, work in the ESC lab focuses on the effects of both acute (single bout) exercise, a physiological stress, and exercise training interventions, a physiological stimulus to induce adaptation, on hematopoietic (HSC) and myogenic stem/progenitor cell (MPC) quantity and function. In both HSCs and MPCs, we have determined that many of the exercise-induced adaptations are mediated primarily by alterations in the local tissue microenvironment, of which, mesenchymal stem/stromal cells (MSCs) are a primary component. We explore these questions using a highly translational approach using in vitro, animal, and human models, and combine molecular/cellular biology techniques with applied functional measures.
Research Themes
- Developing an understanding of the role of exercise in regulating hematopoiesis. Hematopoiesis is the process whereby a rare population of hematopoietic stem cells (HSCs) maintain all blood and immune cell populations through their proliferation and differentiation. Although an extensive body of literature has described the effects of exercise on mature blood and immune cells, only a few studies have examined the effects of exercise on HSCs. During my doctoral training, we demonstrated that exercise could act as a radioprotectant, decreasing genotoxic and cytotoxic damage in hematopoietic cells exposed to a high dose of radiation. We then went on to demonstrate that exercise training could increase HSC content within the bone marrow, and enhance extra-medullary hematopoiesis. Since, HSCs are tightly regulated by their microenvironment within the bone marrow, consisting of a variety of cells that are primarily derived from mesenchymal stromal cells (MSCs), we examined the effects of exercise preconditioning on a pre-clinical model of bone marrow transplantation. We discovered that exercise preconditioning enhance recovery and survival in transplant recipients. My lab has recently completed a study examining the regulation of exercise-induced HSC mobilization in humans and mice with implications for using exercise as a novel adjuvant to traditional mobilization approaches in transplant donors.
- Understanding the physiological role of circulating stem/progenitor cells in tissue repair. Exercise is known to have systemic benefits to a variety of tissues that are not directly stressed by the exercise bout. The mechanisms responsible for the systemic benefits of exercise have not been elucidated. While most attention in this area has been paid to exercise-induced “myokines” or “exerkines” (i.e. paracrine factors released by skeletal muscle in response to contraction that can have systemic effects). A less well-explored area of investigation is the potential for exercise to mobilize stem/progenitor cell populations into circulation to facilitate tissue repair either by differentiation, cell fusion, or by paracrine actions. During my postdoctoral training, I was part of a team that developed a novel in vivo multimodal imaging modality to investigate the role of bone marrow-derived stem/progenitor cells in tissue repair. Work in my lab has identified circulating progenitor cells in overweight and obese children that are related to improved cognitive function, and enhance neurogenesis in vitro.
- Elucidating the role of muscle stem cells in muscle maintenance and adaptation. Maintenance and adaptation of skeletal muscle across the lifespan is dependent upon a population of myogenic stem cells, termed satellite cells. Satellite cells are regulated by soluble factors, extra-cellular matrix (ECM) proteins, and cellular constituents in their microenvironment. Recently, a variety of mesenchymal stem/stromal cell (MSC) populations have been identified in muscle that contribute to muscle adaptation via direct myogenic differentiation, ECM remodeling, and/or paracrine signaling. During my doctoral training, we conducted a series of studies examining the molecular regulation of satellite cells in response to muscle damage and exercise in humans. During my postdoctoral training, we conducted a series of studies that examined the role of MSCs in satellite cell regulation, and determined that MSCs promote satellite cell proliferation in vitro, and contribute to muscle repair and satellite cell expansion in vivo. Recent work in my lab is examining the effects of leucine and high protein foods on satellite cells, and have identified that the leucine transporter, L-type amino acid transporter 1 (LAT1), is differentially regulated during satellite cell activation, proliferation and differentiation. Additionally, we are examining the relationship between satellite cells and MSCs in overweight and obese adults, following radiation exposure in rodents, and in a rodent model of obesity-induced colon cancer and exercise.